Regional expression and cellular localization of the alpha1 and beta subunit of high voltage-activated calcium channels in rat brain
- PMID: 9006977
- PMCID: PMC6793722
- DOI: 10.1523/JNEUROSCI.17-04-01339.1997
Regional expression and cellular localization of the alpha1 and beta subunit of high voltage-activated calcium channels in rat brain
Abstract
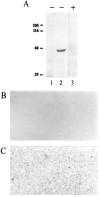
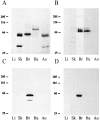
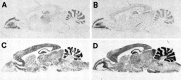
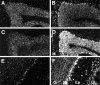
The neuronal high voltage-activated calcium channels are a family of ion channels composed from up to five different alpha1 and four different beta subunits. The neuronal distribution and subunit composition of calcium channels were investigated using subunit-specific antibodies and riboprobes. The beta subunit-specific antibodies identified the presence of beta1a in skeletal muscle; beta2 in heart; and beta2, beta3, and beta4 in brain. The beta3 protein was widely distributed in rat brain, with prominent labeling of olfactory bulb, cortex, hippocampus, and habenula. The beta4 protein was also widely expressed, most prominently in the cerebellum. beta2 protein was expressed at only low levels. In situ hybridization with beta subunit-specific riboprobes confirmed the differential expression pattern of the individual subunits. Hybridization with riboprobes specific for the alpha1A, alpha1B, alpha1C, and alpha1D subunits showed a broad distribution of alpha1A and alpha1B transcripts, whereas the expression level of alpha1C and alpha1D mRNA was lower and more spatially restricted. The overall expression pattern and cellular localization suggested that beta4 may associate predominantly, but probably not exclusively, with the alpha1A subunit, and beta3 with the alpha1B subunit. In certain brain areas such as the habenula, the beta3 subunit may associate with other alpha1 subunits too. Furthermore, the beta2 subunit may form complexes with different alpha1 subunits in brain and cardiac muscle. These results demonstrate that a given beta subunit may associate with different alpha1 subunits in a cell type-dependent manner, contributing to the diversity of the neuronal calcium channels.
Figures




Similar articles
-
beta subunit reshuffling modifies N- and P/Q-type Ca2+ channel subunit compositions in lethargic mouse brain.Mol Cell Neurosci. 1999 Apr;13(4):293-311. doi: 10.1006/mcne.1999.0748. Mol Cell Neurosci. 1999. PMID: 10328888
-
Expression pattern of voltage-dependent calcium channel alpha1 and beta subunits in adrenal gland of N-type Ca2+ channel alpha1B subunit gene-deficient mice.Mol Cell Biochem. 2005 Mar;271(1-2):91-9. doi: 10.1007/s11010-005-5387-8. Mol Cell Biochem. 2005. PMID: 15881659
-
Differential expression and association of calcium channel alpha1B and beta subunits during rat brain ontogeny.J Biol Chem. 1998 Jun 5;273(23):14495-502. doi: 10.1074/jbc.273.23.14495. J Biol Chem. 1998. PMID: 9603963
-
Differential expression and association of calcium channel subunits in development and disease.J Bioenerg Biomembr. 1998 Aug;30(4):409-18. doi: 10.1023/a:1021997924473. J Bioenerg Biomembr. 1998. PMID: 9758336 Review.
-
Structures and functions of calcium channel beta subunits.J Bioenerg Biomembr. 1998 Aug;30(4):357-75. doi: 10.1023/a:1021989622656. J Bioenerg Biomembr. 1998. PMID: 9758332 Review.
Cited by
-
Maturation of rat cerebellar Purkinje cells reveals an atypical Ca2+ channel current that is inhibited by omega-agatoxin IVA and the dihydropyridine (-)-(S)-Bay K8644.J Physiol. 2007 Feb 1;578(Pt 3):693-714. doi: 10.1113/jphysiol.2006.121905. Epub 2006 Nov 23. J Physiol. 2007. PMID: 17124267 Free PMC article.
-
Compensatory regulation of Cav2.1 Ca2+ channels in cerebellar Purkinje neurons lacking parvalbumin and calbindin D-28k.J Neurophysiol. 2010 Jan;103(1):371-81. doi: 10.1152/jn.00635.2009. Epub 2009 Nov 11. J Neurophysiol. 2010. PMID: 19906882 Free PMC article.
-
Voltage-activated calcium channel expression profiles in mouse brain and cultured hippocampal neurons.Neuroscience. 2010 May 19;167(3):786-98. doi: 10.1016/j.neuroscience.2010.02.037. Epub 2010 Feb 24. Neuroscience. 2010. PMID: 20188150 Free PMC article.
-
Nimodipine Reappraised: An Old Drug With a Future.Curr Neuropharmacol. 2020;18(1):65-82. doi: 10.2174/1570159X17666190927113021. Curr Neuropharmacol. 2020. PMID: 31560289 Free PMC article. Review.
-
Aminopyridines potentiate synaptic and neuromuscular transmission by targeting the voltage-activated calcium channel beta subunit.J Biol Chem. 2009 Dec 25;284(52):36453-36461. doi: 10.1074/jbc.M109.075523. Epub 2009 Oct 22. J Biol Chem. 2009. PMID: 19850918 Free PMC article.
References
-
- Ahlijanian MK, Westenbroek RE, Catterall WA. Subunit structure and localization of dihydropyridine-sensitive calcium channels in mammalian brain, spinal cord, and retina. Neuron. 1990;4:819–832. - PubMed
-
- Benke D, Wenzel A, Scheuer L, Fritschy JM, Möhler H. Immunobiochemical characterization of the NMDA–receptor subunit NR1 in the developing and adult rat brain. J Recept Signal Transduct Res. 1995;15:393–411. - PubMed
-
- Blanar MA, Rutter WJ. Interaction cloning: identification of a helix-loop-helix zipper protein that interacts with c-Fos. Science. 1992;256:1014–1018. - PubMed
-
- Castellano A, Perez-Reyes E. Molecular diversity of Ca2+ channel beta subunits. Biochem Soc Trans. 1994;22:483–488. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
