Prism adaptation of reaching movements: specificity for the velocity of reaching
- PMID: 9006989
- PMCID: PMC6793717
- DOI: 10.1523/JNEUROSCI.17-04-01481.1997
Prism adaptation of reaching movements: specificity for the velocity of reaching
Abstract
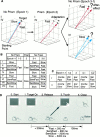
Accurate reaching toward a visual target is disturbed after the visual field is displaced by prisms but recovers with practice. When the prisms are removed, subjects misreach in the direction opposite to the prism displacement (aftereffect). The present study demonstrated that the severity of the aftereffect depends on the velocity of the movements during and after the visual displacement. Trained subjects were required to reach with one of four movement durations (<300, approximately 800, approximately 2000, and approximately 5000 msec) from a fixed starting point to a target that appeared at a random location on a tangent screen (400 mm away). The size of the aftereffect was largest when the movement after the removal was performed with the same duration as that performed with the prisms. It became smaller as the difference in velocity became larger. When the contralateral arm was used after visual displacement, the aftereffect was never significant. Because the adaptation does not generalize across velocities or to the other arm, we infer that the underlying changes occur at a later stage in the transformation from visual input to motor output, in which not only the direction but also the time-dependent parameters of movements, such as velocity, acceleration or force, are represented.
Figures





References
-
- Ashe J, Georgopoulos AP. Movement parameters and neural activity in motor cortex and area 5. Cereb Cortex. 1994;6:590–600. - PubMed
-
- Baily JS. Adaptation to prisms: do proprioceptive changes mediate adapted behaviour with ballistic arm movements? Q J Exp Psychol. 1972;24:8–20. - PubMed
-
- Bizzi E, Mussa-Ivaldi FA, Giszter S. Computations underlying the execution of movement: a biological perspective. Science. 1991;253:287–291. - PubMed
-
- Cheney PD, Fetz EE. Functional classes of primate cortico-motoneuronal cells and their relation to active force. J Neurophysiol. 1980;44:773–791. - PubMed
-
- Flament D, Hore J. Relations of motor cortex neural discharge to kinematics of passive and active elbow movements in the monkey. J Neurophysiol. 1988;60:1268–1284. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
