Chromosomes with two intact axial cores are induced by G2 checkpoint override: evidence that DNA decatenation is not required to template the chromosome structure
- PMID: 9008701
- PMCID: PMC2132461
- DOI: 10.1083/jcb.136.1.29
Chromosomes with two intact axial cores are induced by G2 checkpoint override: evidence that DNA decatenation is not required to template the chromosome structure
Abstract
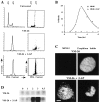
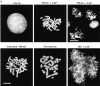
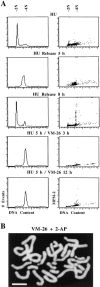
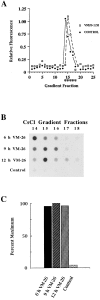
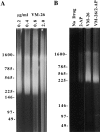
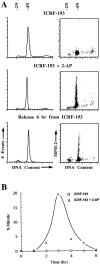
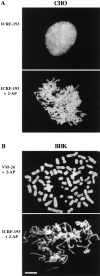
Here we report that DNA decatenation is not a physical requirement for the formation of mammalian chromosomes containing a two-armed chromosome scaffold. 2-aminopurine override of G2 arrest imposed by VM-26 or ICRF-193, which inhibit topoisomerase II (topo II)-dependent DNA decatenation, results in the activation of p34cdc2 kinase and entry into mitosis. After override of a VM-26-dependent checkpoint, morphologically normal compact chromosomes form with paired axial cores containing topo II and ScII. Despite its capacity to form chromosomes of normal appearance, the chromatin remains covalently complexed with topo II at continuous levels during G2 arrest with VM-26. Override of an ICRF-193 block, which inhibits topo II-dependent decatenation at an earlier step than VM-26, also generates chromosomes with two distinct, but elongated, parallel arms containing topo II and ScII. These data demonstrate that DNA decatenation is required to pass a G2 checkpoint, but not to restructure chromatin for chromosome formation. We propose that the chromosome core structure is templated during interphase, before DNA decatenation, and that condensation of the two-armed chromosome scaffold can therefore occur independently of the formation of two intact and separate DNA helices.
Figures



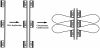
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

