An early stage of membrane fusion mediated by the low pH conformation of influenza hemagglutinin depends upon membrane lipids
- PMID: 9008705
- PMCID: PMC2132452
- DOI: 10.1083/jcb.136.1.81
An early stage of membrane fusion mediated by the low pH conformation of influenza hemagglutinin depends upon membrane lipids
Abstract
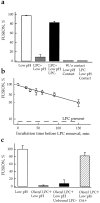
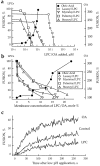
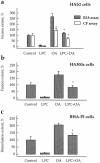
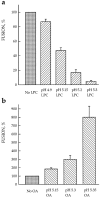
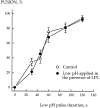
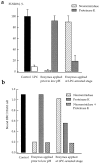
While the specificity and timing of membrane fusion in diverse physiological reactions, including virus-cell fusion, is determined by proteins, fusion always involves the merger of membrane lipid bilayers. We have isolated a lipid-dependent stage of cell-cell fusion mediated by influenza hemagglutinin and triggered by cell exposure to mildly acidic pH. This stage preceded actual membrane merger and fusion pore formation but was subsequent to a low pH-induced change in hemagglutinin conformation that is required for fusion. A low pH conformation of hemagglutinin was required to achieve this lipid-dependent stage and also, downstream of it, to drive fusion to completion. The lower the pH of the medium applied to trigger fusion and, thus, the more hemagglutinin molecules activated, the less profound was the dependence of fusion on lipids. Membrane-incorporated lipids affected fusion in a manner that correlated with their dynamic molecular shape, a characteristic that determines a lipid monolayer's propensity to bend in different directions. The lipid sensitivity of this stage, i.e., inhibition of fusion by inverted cone-shaped lysophosphatidylcholine and promotion by cone-shaped oleic acid, was consistent with the stalk hypothesis of fusion, suggesting that fusion proteins begin membrane merger by promoting the formation of a bent, lipid-involving, stalk intermediate.
Figures

References
-
- Alford D, Ellens H, Bentz J. Fusion of influenza virus with sialic acid-bearing target membranes. Biochemistry. 1994;33:1977–1987. - PubMed
-
- Bhamidipati SP, Hamilton JA. Interactions of lyso 1-palmitoylphosphatidylcholine with phospholipids: a 13C and 31P NMR study. Biochemistry. 1995;34:5666–5677. - PubMed
-
- Broring K, Haest CW, Deuticke B. Translocation of oleic acid across the erythrocyte membrane. Evidence for a fast process. Biochim Biophys Acta. 1989;986:321–331. - PubMed
-
- Bullough PA, Hughson FM, Skehel JJ, Wiley DC. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature (Lond) 1994;371:37–43. - PubMed
-
- Carr CM, Kim PS. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell. 1993;73:823–832. - PubMed

