Phosphoregulation of an inner dynein arm complex in Chlamydomonas reinhardtii is altered in phototactic mutant strains
- PMID: 9008712
- PMCID: PMC2132467
- DOI: 10.1083/jcb.136.1.177
Phosphoregulation of an inner dynein arm complex in Chlamydomonas reinhardtii is altered in phototactic mutant strains
Abstract
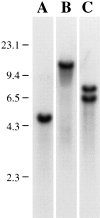
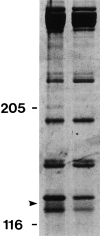
To gain a further understanding of axonemal dynein regulation, mutant strains of Chlamydomonas reinhardtii that had defects in both phototactic behavior and flagellar motility were identified and characterized. ptm1, ptm2, and ptm3 mutant strains exhibited motility phenotypes that resembled those of known inner dynein arm region mutant strains, but did not have biochemical or genetic phenotypes characteristic of other inner dynein arm mutations. Three other mutant strains had defects in the f class of inner dynein arms. Dynein extracts from the pf9-4 strain were missing the entire f complex. Strains with mutations in pf9/ida1, ida2, or ida3 failed to assemble the f dynein complex and did not exhibit phototactic behavior. Fractionated dynein from mia1-1 and mia2-1 axonemes exhibited a novel f class inner dynein arm biochemical phenotype; the 138-kD f intermediate chain was present in altered phosphorylation forms. In vitro axonemal dynein activity was reduced by the mia1-1 and mia2-1 mutations. The addition of kinase inhibitor restored axonemal dynein activity concomitant with the dephosphorylation of the 138-kD f intermediate chain. Dynein extracts from uni1-1 axonemes, which specifically assemble only one of the two flagella, contained relatively high levels of the altered phosphorylation forms of the 138-kD intermediate chain. We suggest that the f dynein complex may be phosphoregulated asymmetrically between the two flagella to achieve phototactic turning.
Figures




Similar articles
-
Two types of Chlamydomonas flagellar mutants missing different components of inner-arm dynein.J Cell Biol. 1991 Feb;112(3):441-7. doi: 10.1083/jcb.112.3.441. J Cell Biol. 1991. PMID: 1825085 Free PMC article.
-
Extragenic suppressors of paralyzed flagellar mutations in Chlamydomonas reinhardtii identify loci that alter the inner dynein arms.J Cell Biol. 1992 Sep;118(5):1163-76. doi: 10.1083/jcb.118.5.1163. J Cell Biol. 1992. PMID: 1387404 Free PMC article.
-
The MIA complex is a conserved and novel dynein regulator essential for normal ciliary motility.J Cell Biol. 2013 Apr 15;201(2):263-78. doi: 10.1083/jcb.201211048. Epub 2013 Apr 8. J Cell Biol. 2013. PMID: 23569216 Free PMC article.
-
Functional diversity of axonemal dyneins as studied in Chlamydomonas mutants.Int Rev Cytol. 2002;219:115-55. doi: 10.1016/s0074-7696(02)19012-7. Int Rev Cytol. 2002. PMID: 12211628 Review.
-
Regulation of dynein-driven microtubule sliding by an axonemal kinase and phosphatase in Chlamydomonas flagella.Cell Motil Cytoskeleton. 1995;32(2):106-9. doi: 10.1002/cm.970320207. Cell Motil Cytoskeleton. 1995. PMID: 8681389 Review.
Cited by
-
Disruption of the A-kinase anchoring domain in flagellar radial spoke protein 3 results in unregulated axonemal cAMP-dependent protein kinase activity and abnormal flagellar motility.Mol Biol Cell. 2006 Jun;17(6):2626-35. doi: 10.1091/mbc.e06-02-0095. Epub 2006 Mar 29. Mol Biol Cell. 2006. PMID: 16571668 Free PMC article.
-
Regulation of flagellar dynein by phosphorylation of a 138-kD inner arm dynein intermediate chain.J Cell Biol. 1997 Jan 13;136(1):167-76. doi: 10.1083/jcb.136.1.167. J Cell Biol. 1997. PMID: 9008711 Free PMC article.
-
A dynein-associated photoreceptor protein prevents ciliary acclimation to blue light.Sci Adv. 2021 Feb 26;7(9):eabf3621. doi: 10.1126/sciadv.abf3621. Print 2021 Feb. Sci Adv. 2021. PMID: 33637535 Free PMC article.
-
CDKL5 regulates flagellar length and localizes to the base of the flagella in Chlamydomonas.Mol Biol Cell. 2013 Mar;24(5):588-600. doi: 10.1091/mbc.E12-10-0718. Epub 2013 Jan 2. Mol Biol Cell. 2013. PMID: 23283985 Free PMC article.
-
Asymmetries in the cilia of Chlamydomonas.Philos Trans R Soc Lond B Biol Sci. 2020 Feb 17;375(1792):20190153. doi: 10.1098/rstb.2019.0153. Epub 2019 Dec 30. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 31884924 Free PMC article. Review.
References
-
- Blum H, Beier H, Gross HJ. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–97.
-
- Brokaw CJ, Kamiya R. Bending patterns of Chlamydomonasflagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil. 1987;8:68–75. - PubMed
-
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature (Lond) 1982;296:613–620. - PubMed

