Nuclear localization of cyclin B1 mediates its biological activity and is regulated by phosphorylation
- PMID: 9012813
- PMCID: PMC19542
- DOI: 10.1073/pnas.94.2.502
Nuclear localization of cyclin B1 mediates its biological activity and is regulated by phosphorylation
Abstract
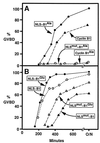
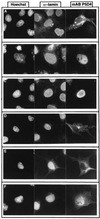
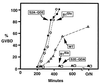
M-phase promoting factor or maturation promoting factor, a key regulator of the G2-->M transition of the cell cycle, is a complex of cdc2 and a B-type cyclin. We have previously shown that Xenopus cyclin B1 has five sites of Ser phosphorylation, four of which map to a recently identified cytoplasmic retention signal (CRS). The CRS appears to be responsible for the cytoplasmic localization of B-type cyclins, although the underlying mechanism is still unclear. Phosphorylation of cyclin B1 is not required for cdc2 binding or cdc2 kinase activity. However, when all of the Ser phosphorylation sites in the CRS are mutated to Ala to abolish phosphorylation, the mutant cyclin B1Ala is inactivated; activity can be enhanced by mutation of these residues to Glu to mimic phosphoserine, suggesting that phosphorylation of cyclin B1 is required for its biological activity. Here we show that biological activity can be restored to cyclin B1Ala by appending either a nuclear localization signal (NLS), or a second CRS domain with the Ser phosphorylation sites mutated to Glu, while fusion of a second CRS domain with the Ser phosphorylation sites mutated to Ala inactivates wild-type cyclin B1. Nuclear histone H1 kinase activity was detected in association with cyclin B1Ala targeted to the nucleus by a wild-type NLS, but not by a mutant NLS. These results demonstrate that nuclear translocation mediates the biological activity of cyclin B1 and suggest that phosphorylation within the CRS domain of cyclin B1 plays a regulatory role in this process. Furthermore, given the similar in vitro substrate specificity of cyclin-dependent kinases, this investigation provides direct evidence for the hypothesis that the control of subcellular localization of cyclins plays a key role in regulating the biological activity of cyclin-dependent kinase-cyclin complexes.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

