Inactivation of FtsI inhibits constriction of the FtsZ cytokinetic ring and delays the assembly of FtsZ rings at potential division sites
- PMID: 9012823
- PMCID: PMC19552
- DOI: 10.1073/pnas.94.2.559
Inactivation of FtsI inhibits constriction of the FtsZ cytokinetic ring and delays the assembly of FtsZ rings at potential division sites
Abstract
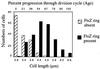
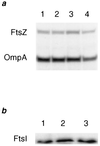
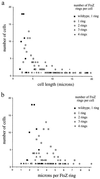
A universally conserved event in cell division is the formation of a cytokinetic ring at the future site of division. In the bacterium Escherichia coli, this ring is formed by the essential cell division protein FtsZ. We have used immunofluorescence microscopy to show that FtsZ assembles early in the division cycle, suggesting that constriction of the FtsZ ring is regulated and supporting the view that FtsZ serves as a bacterial cytoskeleton. Assembly of FtsZ rings was heterogeneously affected in an ftsI temperature-sensitive mutant grown at the nonpermissive temperature, some filaments displaying a striking defect in FtsZ assembly and others displaying little or no defect. By using low concentrations of the beta-lactams cephalexin and piperacillin to specifically inhibit FtsI (PBP3), an enzyme that synthesizes peptidoglycan at the division septum, we show that FtsZ ring constriction requires the transpeptidase activity of FtsI. Unconstricted FtsZ rings are stably trapped at the midpoint of the cell for several generations after inactivation of FtsI, whereas partially constricted FtsZ rings are less effectively trapped. In addition, FtsZ rings are able to assemble in newborn cells in the presence of cephalexin, suggesting that newborn cells contain a site at which FtsZ can assemble (the nascent division site) and that the transpeptidase activity of FtsI is not required for assembly of FtsZ at these sites. However, aside from this first round of FtsZ ring assembly, very few additional FtsZ rings assemble in the presence of cephalexin, even after several generations of growth. One interpretation of these results is that the transpeptidase activity of FtsI is required, directly or indirectly, for the assembly of nascent division sites and thereby for future assembly of FtsZ rings.
Figures

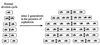
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

