Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice
- PMID: 9012825
- PMCID: PMC19554
- DOI: 10.1073/pnas.94.2.569
Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice
Abstract
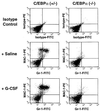
Transcription factors are master regulatory switches of differentiation, including the development of specific hematopoietic lineages from stem cells. Here we show that mice with targeted disruption of the CCAAT enhancer binding protein alpha gene (C/EBP alpha) demonstrate a selective block in differentiation of neutrophils. Mature neutrophils and eosinophils are not observed in the blood or fetal liver of mutant animals, while other hematopoietic lineages, including monocytes, are not affected. Instead, most of the white cells in the peripheral blood of mutant mice had the appearance of myeloid blasts. We also observed a selective loss of expression of a critical gene target of CCAAT enhancer binding protein alpha, the granulocyte colony-stimulating factor receptor. As a result, multipotential myeloid progenitors from the mutant fetal liver are unable to respond to granulocyte colony-stimulating factor signaling, although they are capable of forming granulocyte-macrophage and macrophage colonies in methylcellulose in response to other growth factors. Finally, we demonstrate that the lack of granulocyte development results from a defect intrinsic to the hematopoietic system; transplanted fetal liver from mutant mice can reconstitute lymphoid but not neutrophilic cells in irradiated recipients. These studies suggest a model by which transcription factors can direct the differentiation of multipotential precursors through activation of expression of a specific growth factor receptor, allowing proliferation and differentiation in response to a specific extracellular signal. In addition, the c/ebp alpha -/- mice may be useful in understanding the mechanisms involved in acute myelogenous leukemia, in which a block in differentiation of myeloid precursors is a key feature of the disease.
Figures




References
-
- Shivdasani R A, Orkin S H. Blood. 1996;87:4025–4039. - PubMed
-
- Kehrl J H. Stem Cells. 1995;13:223–241. - PubMed
-
- Nichols J, Nimer S D. Blood. 1992;80:2953–2963. - PubMed
-
- Hromas R, Zon L, Friedman A D. Crit Rev Oncol Hematol. 1992;12:167–190. - PubMed
-
- Shapiro L H, Look A T. Curr Opin Hematol. 1995;2:3–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

