Epidermal growth factor receptor activation induces nuclear targeting of cyclooxygenase-2, basolateral release of prostaglandins, and mitogenesis in polarizing colon cancer cells
- PMID: 9012840
- PMCID: PMC19569
- DOI: 10.1073/pnas.94.2.657
Epidermal growth factor receptor activation induces nuclear targeting of cyclooxygenase-2, basolateral release of prostaglandins, and mitogenesis in polarizing colon cancer cells
Abstract
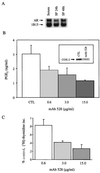
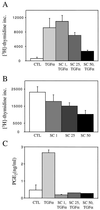
Nonsteroidal antiinflammatory drugs reduce the risk of colon cancer, possibly via cyclooxygenase (COX) inhibition. The growth factor-inducible COX-2, which is overexpressed in neoplastic colonic tissue, is an attractive target to mediate this effect. Herein we have exploited the ability of a human colon cancer cell line, HCA-7 Colony 29, to polarize when cultured on Transwell (Costar) filters to study COX-2 production and the vectorial release of prostaglandins (PGs). Administration of type alpha transforming growth factor to the basolateral compartment, in which the epidermal growth factor receptor (EGFR) resides, results in a marked induction of COX-2 immunoreactivity at the base of the cells and the unexpected appearance of COX-2 in the nucleus. The increase in COX-2 protein is associated with a dose- and time-dependent increase in PG levels in the basolateral, but not apical, medium. Amphiregulin is the most abundantly expressed EGFR ligand in these cells, and the protein is present at the basolateral surface. EGFR blockade reduces baseline COX-2 immunoreactivity, PG levels, and mitogenesis in a concentration-dependent manner. Two specific COX-2 inhibitors, SC-58125 and NS 398, also, in a dose-dependent manner, attenuate baseline and type alpha transforming growth factor-stimulated mitogenesis, although PG levels are decreased > 90% at all concentrations of inhibitor tested. These findings show that activation of the EGFR stimulates COX-2 production and its translocation to the nucleus, vectorial release of PGs, and mitogenesis in polarized HCA-7 Colony 29 cells.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

