Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets
- PMID: 9012841
- PMCID: PMC19570
- DOI: 10.1073/pnas.94.2.663
Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets
Abstract
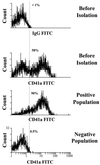
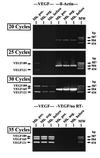
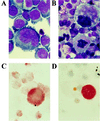
We have shown that coculture of bone marrow microvascular endothelial cells with hematopoietic progenitor cells results in proliferation and differentiation of megakaryocytes. In these long-term cultures, bone marrow microvascular endothelial cell monolayers maintain their cellular integrity in the absence of exogenous endothelial growth factors. Because this interaction may involve paracrine secretion of cytokines, we evaluated megakaryocytic cells for secretion of cytokines, we evaluated megakaryocytic cells for secretion of vascular endothelial growth factor (VEGF). Megakaryocytes (CD41a+) were generated by ex vivo expansion of hematopoietic progenitor cells with kit-ligand and thrombopoietin for 10 days and further purified with immunomagnetic microbeads. Using reverse transcription-PCR, we showed that megakaryocytic cell lines (Dami, HEL) and purified megakaryocytes expressed mRNA of the three VEGF isoforms (121, 165, and 189 amino acids). Large quantities of VEGF (> 1 ng/10(6) cells/3 days) were detected in the supernatant of Dami cells, ex vivo-generated megakaryocytes, and CD41a+ cells isolated from bone marrow. The constitutive secretion of VEGF by CD41a+ cells was stimulated by growth factors of the megakaryocytic lineage (interleukin 3, thrombopoietin). Western blotting of heparin-Sepharose-enriched supernatant mainly detected the isoform VEGF165. In addition, immunohistochemistry showed intracytoplasmic VEGF in polyploid megakaryocytes. Thrombin stimulation of megakaryocytes and platelets resulted in rapid release of VEGF within 30 min. We conclude that human megakaryocytes produce and secrete VEGF in an inducible manner. Within the bone marrow microenvironment, VEGF secreted by megakaryocytes may contribute to the proliferation of endothelial cells. VEGF delivered to sites of vascular injury by activated platelets may initiate angiogenesis.
Figures

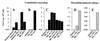


References
-
- Rafii S, Shapiro F, Pettengell R, Ferris B, Nachman R L, Moore M A S, Asch A S. Blood. 1995;86:3353–3363. - PubMed
-
- Tavassoli M, Aoki M. Blood Cells. 1989;15:59–72. - PubMed
-
- Wickenhauser C, Lorenzen J, Thiele J, Hillienhof A, Jungheim K, Schmitz B, Hansmann M, Fischer R. Blood. 1995;85:685–691. - PubMed
-
- Jones C L A, Witte D P, Feller M J, Fugman D A, Dorn G W, Liebermann M A. Biochim Biophys Acta. 1992;1136:272–282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

