Chromosome fragments possessing only one kinetochore can congress to the spindle equator
- PMID: 9015296
- PMCID: PMC2134806
- DOI: 10.1083/jcb.136.2.229
Chromosome fragments possessing only one kinetochore can congress to the spindle equator
Abstract
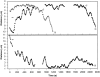
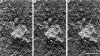
We used laser microsurgery to cut between the two sister kinetochores on bioriented prometaphase chromosomes to produce two chromosome fragments containing one kinetochore (CF1K). Each of these CF1Ks then always moved toward the spindle pole to which their kinetochores were attached before initiating the poleward and away-from-the-pole oscillatory motions characteristic of monooriented chromosomes. CF1Ks then either: (a) remained closely associated with this pole until anaphase (50%), (b) moved (i.e., congressed) to the spindle equator (38%), where they usually (13/19 cells) remained stably positioned throughout the ensuing anaphase, or (c) reoriented and moved to the other pole (12%). Behavior of congressing CF1Ks was indistinguishable from that of congressing chromosomes containing two sister kinetochores. Three-dimensional electron microscopic tomographic reconstructions of CF1Ks stably positioned on the spindle equator during anaphase revealed that the single kinetochore was highly stretched and/or fragmented and that numerous microtubules derived from the opposing spindle poles terminated in its structure. These observations reveal that a single kinetochore is capable of simultaneously supporting the function of two sister kinetochores during chromosome congression and imply that vertebrate kinetochores consist of multiple domains whose motility states can be regulated independently.
Figures








References
-
- Aubin JE, Osborn M, Weber K. Variations in the distribution and migration of centriole duplexes in mitotic PtK2 cells studied by immunofluorescence microscopy. J Cell Sci. 1980;43:177–194. - PubMed
-
- Ault JG, Nicklas RB. Tension, microtubule rearrangements, and the proper distribution of chromosomes in mitosis. Chromosoma (Berl) 1989;98:33–39. - PubMed
-
- Ault JG, Rieder CL. Chromosome mal-orientation and reorientation during mitosis. Cell Motil Cytoskel. 1992;22:155–159. - PubMed
-
- Brinkley BR, Zinkowski RP, Mollon WL, Davis FM, Pisegna MA, Pershouse M, Rao PN. Movement and segregation of kinetochores experimentally detached from mammalian chromosomes. Nature (Lond) 1988;336:251–254. - PubMed
-
- Cassimeris LC, Rieder CL, Salmon ED. Microtubule assembly and kinetochore directional instability in vertebrate monopolar spindles. Implications for the mechanism of chromosome congression. J Cell Sci. 1994;107:285–297. - PubMed

