Biogenesis of the Saccharomyces cerevisiae mating pheromone a-factor
- PMID: 9015298
- PMCID: PMC2134810
- DOI: 10.1083/jcb.136.2.251
Biogenesis of the Saccharomyces cerevisiae mating pheromone a-factor
Abstract
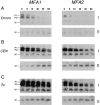
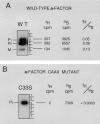
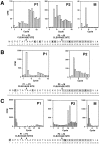
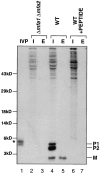
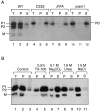
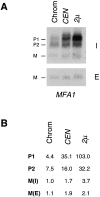
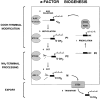
The Saccharomyces cerevisiae mating pheromone a-factor is a prenylated and carboxyl methylated extracellular peptide signaling molecule. Biogenesis of the a-factor precursor proceeds via a distinctive multistep pathway that involves COOH-terminal modification. NH2-terminal proteolysis, and a nonclassical export mechanism. In this study, we examine the formation and fate of a-factor biosynthetic intermediates to more precisely define the events that occur during a-factor biogenesis. We have identified four distinct a-factor biosynthetic intermediates (P0, P1, P2, and M) by metabolic labeling, immunoprecipitation, and SDS-PAGE. We determined the biochemical composition of each by defining their NH2-terminal amino acid and COOH-terminal modification status. Unexpectedly, we discovered that not one, but two NH2-terminal cleavage steps occur during the biogenesis of a-factor. In addition, we have shown that COOH-terminal prenylation is required for the NH2-terminal processing of a-factor and that all the prenylated a-factor intermediates (P1, P2, and M) are membrane bound, suggesting that many steps of a-factor biogenesis occur in association with membranes. We also observed that although the biogenesis of a-factor is a rapid process, it is inherently inefficient, perhaps reflecting the potential for regulation. Previous studies have identified gene products that participate in the COOH-terminal modification (Ram1p, Ram2p, Ste14p), NH2-terminal processing (Ste24p, Axl1p), and export (Ste6p) of a-factor. The intermediates defined in the present study are discussed in the context of these biogenesis components to formulate an overall model for the pathway of a-factor biogenesis.
Figures
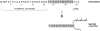




References
-
- Adames N, Blundell K, Ashby MN, Boone C. Role of yeast insulin-degrading enzyme homologs in propheromone processing and bud site selection. Science (Wash DC) 1995;270:464–467. - PubMed
-
- Anderegg RJ, Betz R, Carr SA, Crabb JW, Duntze W. Structure of Saccharomyces cerevisiae mating hormone a-factor. Identification of S-farnesyl cysteine as a structural component. J Biol Chem. 1988;263:18236–18240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

