Docking of yeast vacuoles is catalyzed by the Ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF)
- PMID: 9015302
- PMCID: PMC2134819
- DOI: 10.1083/jcb.136.2.307
Docking of yeast vacuoles is catalyzed by the Ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF)
Abstract
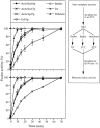
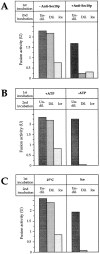
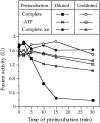
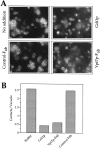
Vacuole inheritance in yeast involves the formation of tubular and vesicular "segregation structures" which migrate into the bud and fuse there to establish the daughter cell vacuole. Vacuole fusion has been reconstituted in vitro and may be used as a model for an NSF-dependent reaction of priming, docking, and fusion. We have developed biochemical and microscopic assays for the docking step of in vitro vacuole fusion and characterized its requirements. The vacuoles must be primed for docking by the action of Sec17p (alpha-SNAP) and Sec18p (NSF). Priming is necessary for both fusion partners. It produces a labile state which requires rapid docking in order to lead productively to fusion. In addition to Sec17p/Sec18p, docking requires the activity of the Ras-like GTPase Ypt7p. Unlike Sec17p/Sec18p, which must act before docking, Ypt7p is directly involved in the docking process itself.
Figures




References
-
- Acharya U, Jacobs R, Peters J-M, Watson N, Farquhar MG, Malhotra V. The formation of golgi stacks from vesiculated golgi membranes requires two distinct fusion events. Cell. 1995;82:895–904. - PubMed
-
- Beckers CJM, Block MR, Glick BS, Rothman JE, Balch WE. Vesicular transport between the endoplasmic reticulum and the golgi stack requires the NEM sensitive fusion protein. Nature (Lond) 1989;339:397–398. - PubMed
-
- Brennwald P, Kearns B, Champion K, Keränen S, Bankaitis V, Novick P. Sec9 is a SNAP-25 like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 1994;79:245–258. - PubMed
-
- Clary DO, Griff IC, Rothman JE. SNAPs, a family of NSF attachment proteins involved in intracellular membrane fusion in animals and yeast. Cell. 1990;61:709–721. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

