Dynamic properties of an inositol 1,4,5-trisphosphate- and thapsigargin-insensitive calcium pool in mammalian cell lines
- PMID: 9015306
- PMCID: PMC2134824
- DOI: 10.1083/jcb.136.2.355
Dynamic properties of an inositol 1,4,5-trisphosphate- and thapsigargin-insensitive calcium pool in mammalian cell lines
Abstract
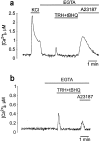
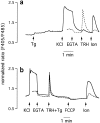
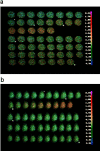
The functional characteristics of a nonacidic, inositol 1,4,5-trisphosphate- and thapsigargin-insensitive Ca2+ pool have been characterized in mammalian cells derived from the rat pituitary gland (GH3, GC, and GH3B6), the adrenal tissue (PC12), and mast cells (RBL-1). This Ca2+ pool is released into the cytoplasm by the Ca2+ ionophores ionomycin or A23187 after the discharge of the inositol 1,4,5-trisphosphate-sensitive store with an agonist coupled to phospholipase C activation and/or thapsigargin. The amount of Ca2+ trapped within this pool increased significantly after a prolonged elevation of intracellular Ca2+ concentration elicited by activation of Ca2+ influx. This pool was affected neither by caffeine-ryanodine nor by mitochondrial uncouplers. Probing mitochondrial Ca2+ with recombinant aequorin confirmed that this pool did not coincide with mitochondria, whereas its homogeneous distribution across the cytosol, as revealed by confocal microscopy, and its insensitivity to brefeldin A make localization within the Golgi complex unlikely. A proton gradient as the driving mechanism for Ca2+ uptake was excluded since ionomycin is inefficient in releasing Ca2+ from acidic pools and Ca2+ accumulation/release in/from this store was unaffected by monensin or NH4Cl, drugs known to collapse organelle acidic pH gradients. Ca2+ sequestration inside this pool, thus, may occur through a low-affinity, high-capacity Ca2+-ATPase system, which is, however, distinct from classical endosarcoplasmic reticulum Ca2+-ATPases. The cytological nature and functional role of this Ca2+ storage compartment are discussed.
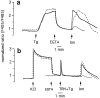
Figures


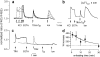


References
-
- Amundson J, Clapham D. Calcium waves. Curr Opin Neurobiol. 1993;3:375–382. - PubMed
-
- Bastianutto C, Clementi E, Codazzi F, Podini P, De Giorgi F, Rizzuto R, Meldolesi J, Pozzan T. Overexpression of calreticulin increases the Ca2+ capacity of rapidly exchanging Ca2+stores and reveals aspects of their lumenal microenvironment and function. J Cell Biol. 1995;130:847–855. - PMC - PubMed
-
- Bian J, Ghosh TK, Wang JC, Gill DL. Identification of intracellular calcium pools. Selective modification by thapsigargin. J Biol Chem. 1991;266:8801–8806. - PubMed
-
- Bobe R, Bredoux R, Wuytack F, Quarck R, Kovacs T, Papp B, Corvazier E, Magnier C, Enouf J. The rat platelet 97-kDa Ca2+ATPase isoform is the sarcoendoplasmic reticulum Ca2+ATPase 3 protein. J Biol Chem. 1994;269:1417–1424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

