NGF and neurotrophin-3 both activate TrkA on sympathetic neurons but differentially regulate survival and neuritogenesis
- PMID: 9015308
- PMCID: PMC2134821
- DOI: 10.1083/jcb.136.2.375
NGF and neurotrophin-3 both activate TrkA on sympathetic neurons but differentially regulate survival and neuritogenesis
Abstract
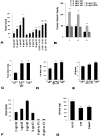
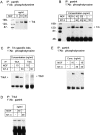
In this report we examine the biological and molecular basis of the control of sympathetic neuron differentiation and survival by NGF and neurotrophin-3 (NT-3). NT-3 is as efficient as NGF in mediating neuritogenesis and expression of growth-associated genes in NGF-dependent sympathetic neurons, but it is 20-40-fold less efficient in supporting their survival. Both NT-3 and NGF induce similar sustained, long-term activation of TrkA, while NGF is 10-fold more efficient than NT-3 in mediating acute, short-term TrkA activity. At similar acute levels of TrkA activation, NT-3 still mediates neuronal survival two- to threefold less well than NGF. However, a mutant NT-3 that activates TrkC, but not TrkA, is unable to support sympathetic neuron survival or neuritogenesis, indicating that NT-3-mediated TrkA activation is necessary for both of these responses. On the basis of these data, we suggest that NGF and NT-3 differentially regulate the TrkA receptor both with regard to activation time course and downstream targets, leading to selective regulation of neuritogenesis and survival. Such differential responsiveness to two ligands acting through the same Trk receptor has important implications for neurotrophin function throughout the nervous system.
Figures




References
-
- Acheson A, Barker PA, Alderson RF, Miller FD, Murphy RA. Detection of brain-derived neurotrophic factor-like activity in fibroblasts and Schwann cells: inhibition by antibodies to NGF. Neuron. 1991;7:265–275. - PubMed
-
- Barbacid M. The trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. - PubMed
-
- Barker PA, Shooter EM. Disruption of NGF binding to the low affinity neurotrophin receptor p75LNTR reduces NGF binding to TrkA on PC12 cells. Neuron. 1994;13:203–215. - PubMed
-
- Barker PA, Lomen-Hoerth C, Gensch EM, Meakin SO, Glass DJ, Shooter EM. Tissue-specific alternative splicing generates two isoforms of the trkA receptor. J Biol Chem. 1993;268:15150–15157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

