Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus
- PMID: 9016870
- PMCID: PMC2196125
- DOI: 10.1084/jem.185.2.207
Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus
Abstract
Mice with X-linked chronic granulomatous disease (CGD) generated by targeted disruption of the gp91phox subunit of the NADPH-oxidase complex (X-CGD mice) were examined for their response to respiratory challenge with Aspergillus fumigatus. This opportunistic fungal pathogen causes infection in CGD patients due to the deficient generation of neutrophil respiratory burst oxidants important for damaging A. fumigatus hyphae. Alveolar macrophages from X-CGD mice were found to kill A. fumigatus conidia in vitro as effectively as alveolar macrophages from wild-type mice. Pulmonary disease in X-CGD mice was observed after administration of doses ranging from 10(5) to 48 spores, none of which produced disease in wild-type mice. Higher doses produced a rapidly fatal bronchopneumonia in X-CGD mice, whereas progression of disease was slower at lower doses, with development of chronic inflammatory lesions. Marked differences were also observed in the response of X-CGD mice to the administration of sterilized Aspergillus hyphae into the lung. Within 24 hours of administration, X-CGD mice had significantly higher numbers of alveolar neutrophils and increased expression of the proinflammatory cytokines IL-1 beta and TNF-alpha relative to the responses seen in wild-type mice. By one week after administration, pulmonary inflammation was resolving in wild-type mice, whereas X-CGD mice developed chronic granulomatous lesions that persisted for at least six weeks. This is the first experimental evidence that chronic inflammation in CGD does not always result from persistent infection, and suggests that the clinical manifestations of this disorder reflect both impaired microbial killing as well as other abnormalities in the inflammatory response in the absence of a respiratory burst.
Figures













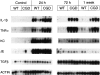
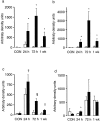
References
-
- Dinauer MC. The respiratory burst oxidase and the molecular genetics of chronic granulomatous disease. Crit Rev Clin Lab Sci. 1993;30:329–369. - PubMed
-
- Carson MJ, Chadwick DL, Brubaker CA, Cleland RS, Landing BH. Thirteen boys with progressive septic granulomatosis. Pediatrics. 1965;35:405–412. - PubMed
-
- Johnston RB, Jr, McMurry J. Chronic familial granulomatosis. Am J Dis Child. 1967;114:370–378. - PubMed
-
- Gallin JI, Buescher ES, Seligmann BE, Nath J, Gaither T, Katz P. Recent advances in chronic granulomatous disease. Ann Intern Med. 1983;99:657–674. - PubMed
-
- Moskaluk CA, Pogrebniak HW, Pass HI, Gallin JI, Travis WD. Surgical pathology of the lung in chronic granulomatous disease. Am J Clin Pathol. 1994;102:684–691. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

