Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures
- PMID: 9016880
- PMCID: PMC2196118
- DOI: 10.1084/jem.185.2.317
Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures
Abstract
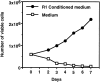
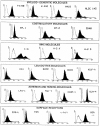
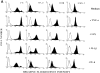
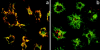
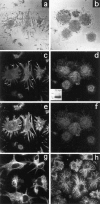
The signals controlling the checkpoints of dendritic cells (DC) maturation and the correlation between phenotypical and functional maturational stages were investigated in a defined model system of growth factor-dependent immature mouse DC. Three sequential stages of DC maturation (immature, mature, and apoptotic) were defined and characterized. Immature DC (stage 1) had low expression of costimulatory molecules, highly organized cytoskeleton, focal adhesion plaques, and slow motility; accordingly, they were very efficient in antigen uptake and processing of soluble proteins. Further, at this stage most of major histocompatibility complex class II molecules were within cytoplasmic compartments consistent with a poor allostimulatory capacity. Bacteria or cytokines were very efficient in inducing progression from stage 1 towards stage 2 (mature). Morphological changes were observed by confocal analysis including depolymerization of F-actin and loss of vinculin containing adhesive structures which correlates with acquisition of high motility. Antigen uptake and presentation of native protein antigen was reduced. In contrast, presentation of immunogenic peptides and allostimulatory activity became very efficient and secretion of IL-12 p75 was detectable after antigen presentation. This functional DC maturation ended by apoptotic cell death, and no reversion to the immature phenotype was observed.
Figures



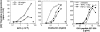

References
-
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

