Stochastic mechanisms in gene expression
- PMID: 9023339
- PMCID: PMC19596
- DOI: 10.1073/pnas.94.3.814
Stochastic mechanisms in gene expression
Abstract
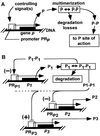
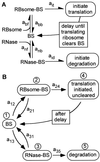
In cellular regulatory networks, genetic activity is controlled by molecular signals that determine when and how often a given gene is transcribed. In genetically controlled pathways, the protein product encoded by one gene often regulates expression of other genes. The time delay, after activation of the first promoter, to reach an effective level to control the next promoter depends on the rate of protein accumulation. We have analyzed the chemical reactions controlling transcript initiation and translation termination in a single such "genetically coupled" link as a precursor to modeling networks constructed from many such links. Simulation of the processes of gene expression shows that proteins are produced from an activated promoter in short bursts of variable numbers of proteins that occur at random time intervals. As a result, there can be large differences in the time between successive events in regulatory cascades across a cell population. In addition, the random pattern of expression of competitive effectors can produce probabilistic outcomes in switching mechanisms that select between alternative regulatory paths. The result can be a partitioning of the cell population into different phenotypes as the cells follow different paths. There are numerous unexplained examples of phenotypic variations in isogenic populations of both prokaryotic and eukaryotic cells that may be the result of these stochastic gene expression mechanisms.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

