Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease
- PMID: 9023366
- PMCID: PMC19623
- DOI: 10.1073/pnas.94.3.967
Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease
Abstract
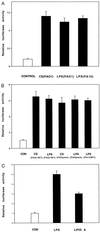
An unresolved question in cystic fibrosis (CF) research is how mutations of the CF transmembrane conductance regulator, a Cl ion channel, cause airway mucus obstruction leading to fatal lung disease. Recent evidence has linked the CF transmembrane conductance regulator mutation to the onset and persistence of Pseudomonas aeruginosa infection in the airways, and here we provide evidence directly linking P. aeruginosa infection to mucus overproduction. We show that P. aeruginosa lipopolysaccharide profoundly upregulates transcription of the mucin gene MUC 2 in epithelial cells via inducible enhancer elements and that this effect is blocked by the tyrosine kinase inhibitors genistein and tyr-phostin AG 126. These findings improve our understanding of CF pathogenesis and suggest that the attenuation of mucin production by lipopolysaccharide antagonists and tyrosine kinase inhibitors could reduce morbidity and mortality in this disease.
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

