Small, membrane-bound, alternatively spliced forms of ankyrin 1 associated with the sarcoplasmic reticulum of mammalian skeletal muscle
- PMID: 9024692
- PMCID: PMC2134284
- DOI: 10.1083/jcb.136.3.621
Small, membrane-bound, alternatively spliced forms of ankyrin 1 associated with the sarcoplasmic reticulum of mammalian skeletal muscle
Abstract
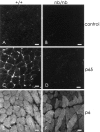
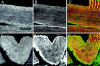
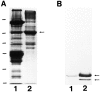
We have recently found that the erythroid ankyrin gene, Ank1, expresses isoforms in mouse skeletal muscle, several of which share COOH-terminal sequence with previously known Ank1 isoforms but have a novel, highly hydrophobic 72-amino acid segment at their NH2 termini. Here, through the use of domain-specific peptide antibodies, we report the presence of the small ankyrins in rat and rabbit skeletal muscle and demonstrate their selective association with the sarcoplasmic reticulum. In frozen sections of rat skeletal muscle, antibodies to the spectrin-binding domain (anti-p65) react only with a 210-kD Ank1 and label the sarcolemma and nuclei, while antibodies to the COOH terminus of the small ankyrin (anti-p6) react with peptides of 20 to 26 kD on immunoblots and decorate the myoplasm in a reticular pattern. Mice homozygous for the normoblastosis mutation (gene symbol nb) are deficient in the 210-kD ankyrin but contain normal levels of the small ankyrins in the myoplasm. In nb/nb skeletal muscle, anti-p65 label is absent from the sarcolemma, whereas anti-p6 label shows the same distribution as in control skeletal muscle. In normal skeletal muscle of the rat, anti-p6 decorates Z lines, as defined by antidesmin distribution, and is also present at M lines where it surrounds the thick myosin filaments. Immunoblots of the proteins isolated with rabbit sarcoplasmic reticulum indicate that the small ankyrins are highly enriched in this fraction. When expressed in transfected HEK 293 cells, the small ankyrins are distributed in a reticular pattern resembling the ER if the NH2-terminal hydrophobic domain is present, but they are uniformly distributed in the cytosol if this domain is absent. These results suggest that the small ankyrins are integral membrane proteins of the sarcoplasmic reticulum. We propose that, unlike the 210-kD form of Ank1, previously localized to the sarcolemma and believed to be a part of the supporting cytoskeleton, the small Ank1 isoforms may stabilize the sarcoplasmic reticulum by linking it to the contractile apparatus.
Figures
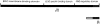






References
-
- Bennett V. Purification of an active proteolytic fragment of the membrane attachment site for human erythrocyte spectrin. J Biol Chem. 1978;253:2292–2299. - PubMed
-
- Bennett V, Gilligan DM. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. - PubMed
-
- Birkenmeier CS, White RA, Peters LL, Hall EJ, Lux SE, Barker JE. Complex patterns of sequence variation and multiple 5′ and 3′ ends are found among transcripts of the erythroid ankyrin gene. J Biol Chem. 1993;268:9533–9540. - PubMed
-
- Bodine DM, Birkenmeier CS, Barker JE. Spectrin deficient inherited hemolytic anemias in the mouse: characterization by spectrin synthesis and mRNA activity in reticulocytes. Cell. 1984;37:721–729. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

