Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility
- PMID: 9024701
- PMCID: PMC2134287
- DOI: 10.1083/jcb.136.3.729
Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility
Abstract
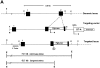
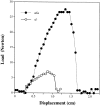
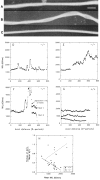
Decorin is a member of the expanding group of widely distributed small leucine-rich proteoglycans that are expected to play important functions in tissue assembly. We report that mice harboring a targeted disruption of the decorin gene are viable but have fragile skin with markedly reduced tensile strength. Ultrastructural analysis revealed abnormal collagen morphology in skin and tendon, with coarser and irregular fiber outlines. Quantitative scanning transmission EM of individual collagen fibrils showed abrupt increases and decreases in mass along their axes. thereby accounting for the irregular outlines and size variability observed in cross-sections. The data indicate uncontrolled lateral fusion of collagen fibrils in the decorindeficient mice and provide an explanation for the reduced tensile strength of the skin. These findings demonstrate a fundamental role for decorin in regulating collagen fiber formation in vivo.
Figures

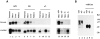





References
-
- Andrikopoulos K, Liu X, Keene DR, Jaenisch R, Ramirez F. Targeted mutation in the col5a2gene reveals a regulatory role for type V collagen during matrix assembly. Nat Genet. 1995;9:31–36. - PubMed
-
- Baribault H, Price J, Miyai K, Oshima RG. Mid-gestational lethality in mice lacking keratin 8. Genes & Dev. 1993;7:1191–1202. - PubMed
-
- Baribault H, Penner J, Iozzo RV, Wilson-Heiner M. Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes & Dev. 1994;8:2964–2973. - PubMed
-
- Bavinton JH, Peters DE, Ramshaw AM. A morphologic study of a mild form of ovine dermatosparaxis. J Invest Dermatol. 1985;84:391–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

