Ectopic alpha2-adrenoceptors couple to N-type Ca2+ channels in axotomized rat sensory neurons
- PMID: 9030623
- PMCID: PMC6573393
- DOI: 10.1523/JNEUROSCI.17-05-01633.1997
Ectopic alpha2-adrenoceptors couple to N-type Ca2+ channels in axotomized rat sensory neurons
Abstract
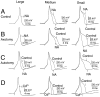
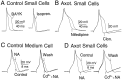
Dorsal root ganglion (DRG) neurons from control rats or from rats in which the sciatic nerve had been sectioned were studied by whole-cell recording techniques. Noradrenaline (10-100 micro;M) activated beta-adrenoceptors and increased L-type Ca2+ channel current in control DRG cells, but this had little effect on excitability (the number of action potentials generated by a pulse of current at rheobasic strength). By contrast, in cells from nerve-damaged animals, noradrenaline activated alpha2-adrenoceptors, suppressed N-type Ca2+ channel current, and increased excitability. In axotomized cells, it also reduced total outward current recorded at +70 mV. Because noradrenaline did not affect total outward current recorded in the presence of the Ca2+ channel blocker Cd2+ (0. 5-1 mM), its effects on excitability may result from reduction of Ca2+-sensitive K+-conductance(s) following suppression of N-type Ca2+ channel current. The strongest effects of noradrenaline were seen in small cells and in cells from animals that exhibited autotomy, a self-mutilatory behavior that can accompany peripheral nerve damage. Because many of these small DRG cells may be involved in the transmission of nociceptive information, changes in coupling between Ca2+ channels and adrenoceptors may contribute to the generation of the ectopic sensory nerve activity that has been implicated in the etiology of neuropathic pain.
Figures




References
-
- Akins PT, McCleskey EW. Characterization of potassium currents in adult rat sensory neurons and modulation by opioids and cyclic AMP. Neuroscience. 1993;56:759–769. - PubMed
-
- Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibres to noxious stimuli. J Neurophysiol. 1969;32:1025–1043. - PubMed
-
- Bonica JJ. Causalgia and other reflex sympathetic dystrophies. In: Bonica JJ, editor. The management of pain. Lea and Febiger; Philadelphia: 1990. pp. 220–243.
-
- Coderre TJ, Grimes RW, Melzak R. Deafferentation and chronic pain in animals: an evaluation of evidence suggesting autotomy is related to pain. Pain. 1986;26:61–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
