Nuclear integration of JAK/STAT and Ras/AP-1 signaling by CBP and p300
- PMID: 9037008
- PMCID: PMC19746
- DOI: 10.1073/pnas.94.4.1074
Nuclear integration of JAK/STAT and Ras/AP-1 signaling by CBP and p300
Abstract
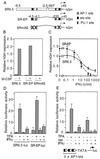
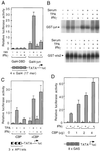
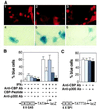
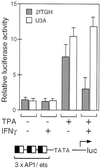
We report that interferon gamma (IFN-gamma) inhibits transcription of the macrophage scavenger receptor gene by antagonizing the Ras-dependent activities of AP-1 and cooperating ets domain transcription factors, apparently as a result of competition between AP-1/ets factors and activated STAT1 for limiting amounts of CBP and p300. Consistent with this model, STAT1 alpha interacts directly with CBP in cells, and microinjection of anti-CBP and anti-p300 antibodies blocks transcriptional responses to IFN-gamma. Cells lacking STAT1 fail to inhibit AP-1/ets activity, and overexpression of CBP both potentiates IFN-gamma-dependent transcription and relieves AP-1/ets repression. Thus, CBP and p300 integrate both positive and negative effects of IFN-gamma on gene expression by serving as essential coactivators of STAT1 alpha, modulating gene-specific responses to simultaneous activation of two or more signal transduction pathways.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

