In vivo control of respiration by cytochrome c oxidase in wild-type and mitochondrial DNA mutation-carrying human cells
- PMID: 9037024
- PMCID: PMC19762
- DOI: 10.1073/pnas.94.4.1166
In vivo control of respiration by cytochrome c oxidase in wild-type and mitochondrial DNA mutation-carrying human cells
Abstract
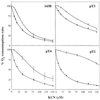
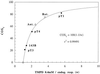
The metabolic control of respiration is still poorly understood, due mainly to the lack of suitable approaches for studying it in vivo. Experiments on isolated mammalian mitochondria have indicated that a relatively small fraction of each of several components of the electron transport chain is sufficient to sustain a normal O2 consumption rate. These experiments, however, may not reflect accurately the in vivo situation, due to the lack in the mitochondrial fraction of essential cytosolic components and to the use of excess of substrates in the in vitro assays. An approach is described here whereby the control of respiration by cytochrome c oxidase (COX; EC 1.9.3.1) was analyzed in intact cultured human osteosarcoma 143B.TK- cells and other wild-type cells and in mitochondrial DNA mutation-carrying human cell lines. Surprisingly, in wild-type cells, only a slightly higher COX capacity was detected than required to support the endogenous respiration rate, pointing to a tighter in vivo control of respiration by COX than generally assumed. Cell lines carrying the MERRF mitochondrial tRNA(Lys) gene mutation, which causes a pronounced decrease in mitochondrial protein synthesis and respiration rates, revealed, in comparison, a significantly greater COX capacity relative to the residual endogenous respiration rate, and, correspondingly, a higher COX inhibition threshold above which the overall respiratory flux was affected. The observed relationship between COX respiratory threshold and relative COX capacity and the potential extension of the present analysis to other respiratory complexes have significant general implications for understanding the pathogenetic role of mutations in mtDNA-linked diseases and the tissue specificity of the mutation-associated phenotype.
Figures


References
-
- Kacser H, Burns J A. In: Rate Control of Biological Processes. Davies D D, editor. Cambridge, U.K.: Cambridge Univ. Press; 1973. pp. 65–104.
-
- Heinrich R, Rapoport T A. Eur J Biochem. 1974;42:89–95. - PubMed
-
- Groen A K, Wanders R J A, Westerhoff H V, Van der Meer R, Tager J M. J Biol Chem. 1982;257:2754–2757. - PubMed
-
- Letellier T, Malgat M, Mazat J-P. Biochim Biophys Acta. 1993;1141:58–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

