Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets
- PMID: 9037030
- PMCID: PMC19768
- DOI: 10.1073/pnas.94.4.1200
Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets
Abstract
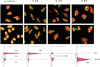
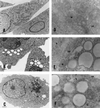
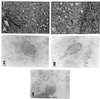
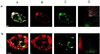
There is now abundant evidence to substantiate an important role of hepatitis C virus (HCV) core protein in cellular gene expression as well as in the viral cycle. Thus the subcellular localization of this protein has important implications. However, several studies have shown controversial results: the HCV core has been, indeed, described as cytoplasmic or nuclear depending on the size of the protein or on the genotype analyzed. We have studied the localization of the HCV core protein in two different cell lines, one nonhepatic (CHO) and the other hepatic (HepG2). Double immunofluorescence staining using a nuclear membrane marker and confocal analysis showed the core protein pattern to be cytoplasmic and globular. This pattern is not cell cycle-regulated. Electron microscopy analysis revealed the nature of the globular staining observed in immunofluorescence. The HCV core protein accumulated at the surface of lipid droplets that were also the unique morphological feature of nonhepatic core transfected cells. The lipid droplets were isolated by sequential ultracentrifugation on the basis of their density; biochemical analysis revealed a prevalence of triglycerides. In addition the core protein colocalized with apolipoprotein AII at the surface of the lipid droplets as revealed by confocal microscopy. Moreover analysis of liver biopsies from chronically HCV-infected chimpanzees revealed that HCV core is cytoplasmic and localized on the endoplasmic reticulum and on lipid droplets. These results clearly define the subcellular localization of the HCV core protein and suggest a relationship between the expression of the HCV core protein and cellular lipid metabolism.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
