Highly purified CD25- resting T cells cannot be infected de novo with HIV-1
- PMID: 9037058
- PMCID: PMC19796
- DOI: 10.1073/pnas.94.4.1361
Highly purified CD25- resting T cells cannot be infected de novo with HIV-1
Abstract
Previous studies have demonstrated that the expression of CD25 can distinguish CD25- latently infected cells from CD25+ cells actively producing virus. Our studies were designed to characterize the nature and stability of the viral genome in CD25- quiescent HIV-1-infected cells and to determine whether these cells could be infected de novo with HIV-1. Our results show that: (i) When unfractionated peripheral blood mononuclear cells are first infected with HIV-1 and the CD25- cells then isolated, the latter contain only incomplete DNA transcripts and no full-length DNA or 2-LTR circles. Phytohemagglutinin activation of these CD25- cells results in the generation of full-length viral DNA and p24 production. (ii) When CD25- CD4+ cells are first purified from peripheral blood mononuclear cells and then incubated with HIV-1, viral DNA cannot be detected, suggesting that these purified cells cannot be infected. Furthermore, CD25-adherent cells do not facilitate the infection of CD4+ CD25- T cells when they were present at the time of incubation with HIV-1. Taken together, these studies suggest either that (i) the CD25- cells containing incomplete DNA transcripts are derived from infected-activated CD25+ cells, which subsequently become CD25- or (ii) the presence of CD25+ cells is required for the infection of CD25- cells in vitro.
Figures
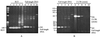



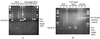

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

