Clade B-based HIV-1 vaccines elicit cross-clade cytotoxic T lymphocyte reactivities in uninfected volunteers
- PMID: 9037064
- PMCID: PMC19802
- DOI: 10.1073/pnas.94.4.1396
Clade B-based HIV-1 vaccines elicit cross-clade cytotoxic T lymphocyte reactivities in uninfected volunteers
Abstract
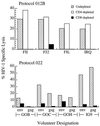
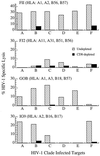
A fundamental goal of current strategies to develop an efficacious vaccine for AIDS is the elicitation of broadly reactive cytotoxic T lymphocyte (CTL) reactivities capable of destroying virally infected targets. Recent application of recombinant canarypox ALVAC/HIV-1 vectors as vaccine immunogens in HIV-1,-noninfected volunteers has produced CTL responses in a significant number of vaccinees. Using a newly developed targeting strategy, we examined the capacity of vaccine-induced CTL to lyse autologous targets infected with a diverse group of viral isolates. CTL derived from recipients of a canarypox ALVAC/HIV-1 gp160 (MN) vaccine were found capable of lysing autologous CD4+ lymphoblasts infected with the prototypic LAI strain of HIV-1. When tested against autologous targets infected with primary HIV-1 isolates representing genetically diverse viral clades, CTL from ALVAC/gp160 recipients showed both a broad pattern of cytolysis in which viruses from all clades tested were recognized as well as a highly restricted pattern in which no primary isolates, including clade B, were lysed. Differences in the HLA haplotypes of the volunteers immunized with the envelope vector might be a major determinant of the relative breadth of their CTL response. In contrast to ALVAC/gp160 vaccinees, recipients of the ALVAC/HIV-1 immunogen containing envelope as well as gag and protease genes consistently had CTL reactivities effective against a spectrum of primary isolate-infected targets. These studies demonstrate for the first time that clade B-based canarypox vaccines can elicit broad CTL reactivities capable of recognizing viruses belonging to genetically diverse HIV-1 clades. The results also reinforce the impact of viral core elements in the vaccine as well as the pattern of major histocompatibility complex class I allelic expression by the vaccine recipient in determining the relative breadth of the cellular response.
Figures

References
-
- Mascola J R, Snyder S W, Weislow O S, Belay S M, Belshe R B, Schwartz D H, Clements M, Dolin R, Graham B S, Gorse G J, Keefer M C, McElrath M J, Walker M C, Wagner K F, McNeil J G, McCutchan F E, Burke D S. J Infect Dis. 1996;173:340–348. - PubMed
-
- Van Cott T C, Bethke F R, Burke D S, Redfield R R, Birx D L. J Immunol. 1995;155:4100–4110. - PubMed
-
- McMichael A, Walker B. AIDS. 1994;8:S155–S173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

