Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor
- PMID: 9037072
- PMCID: PMC19810
- DOI: 10.1073/pnas.94.4.1441
Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor
Abstract
The mechanisms that initiate liver regeneration after resection of liver tissue are not known. To determine whether cytokines are involved in the initiation of liver growth, we studied the regeneration of the liver after partial hepatectomy (PH) in mice lacking type I tumor necrosis factor receptor (TNFR-I). DNA synthesis after PH was severely impaired in these animals, and the expected increases in the binding of the NF-kappaB and STAT3 transcription factors shortly after PH failed to occur. Binding of AP-1 after PH was decreased in TNFR-I knockout mice compared with animals with the intact receptor whereas C/EBP binding was not modified. Injection of interleukin 6 in TNFR-I-deficient animals 30 min before PH corrected the defect in DNA synthesis and restored STAT3 and AP-1 binding to normal levels but had no effect on NF-kappaB binding in the regenerating liver. The results indicate that TNF, signaling through the TNFR-I, can initiate liver regeneration and acts by activating an interleukin 6-dependent pathway that involves the STAT3 transcription factor.
Figures
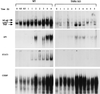

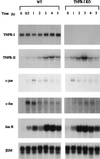
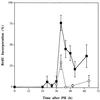

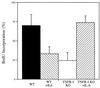
References
-
- Fausto N, Webber E M. In: The Liver Biology and Pathobiology. Arias I, Boyer J, Fausto N, Jakoby W, Schachter D, Shafritz D, editors. New York: Raven; 1994. pp. 1059–1084.
-
- Bucher N L R. In: Liver Regeneration and Carcinogenesis: Molecular and Cellular Mechanisms. Jirtle R L, editor. San Diego: Academic; 1995. pp. 1–25.
-
- Fausto N, Laird A D, Webber E M. FASEB J. 1995;9:1527–1536. - PubMed
-
- Diehl A M, Rai R M. FASEB J. 1996;10:215–227. - PubMed
-
- Taub R. FASEB J. 1996;10:413–427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

