Actions of Ca2+ on an early stage in phototransduction revealed by the dynamic fall in Ca2+ concentration during the bright flash response
- PMID: 9041444
- PMCID: PMC2220062
- DOI: 10.1085/jgp.109.2.141
Actions of Ca2+ on an early stage in phototransduction revealed by the dynamic fall in Ca2+ concentration during the bright flash response
Abstract
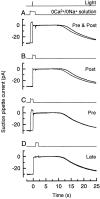
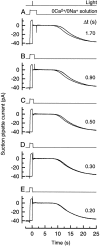
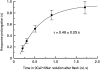
To study the actions of Ca2+ on "early" stages of the transduction cascade, changes in cytoplasmic calcium concentration (Ca2+i) were opposed by manipulating Ca2+ fluxes across the rod outer segment membrane immediately following a bright flash. If the outer segment was exposed to 0 Ca2+/0 Na+ solution for a brief period immediately after the flash, then the period of response saturation was prolonged in comparison with that in Ringer solution. But if the exposure to 0 Ca2+/0Na+ solution instead came before or was delayed until 1 s after the flash then it had little effect. The degree of response prolongation increased with the duration of the exposure to 0 Ca2+/0 Na+ solution, revealing a time constant of 0.49 +/- 0.03 s. By the time the response begins to recover from saturation, Ca2+i seems likely to have fallen to a similar level in each case. Therefore the prolongation of the response when Ca2+i was prevented from changing immediately after the flash seems likely to reflect the abolition of actions of the usual dynamic fall in Ca2+i on an early stage in the transduction cascade at a site which is available for only a brief period after the flash. One possibility is that the observed time constant corresponds to the phosphorylation of photoisomerized rhodopsin.
Figures
References
-
- Cervetto L, Lagnado L, Perry RJ, Robinson DW, McNaughton PA. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature (Lond) 1989;337:740–743. - PubMed
-
- Chen CK, Inglese J, Lefkowitz RJ, Hurley JB. Ca2+-dependent interaction of recoverin with rhodopsin kinase. J Biol Chem. 1995;270:18060–18066. - PubMed
-
- Gray-Keller MP, Detwiler PB. The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron. 1994;13:849–861. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

