A single morphogenetic field gives rise to two retina primordia under the influence of the prechordal plate
- PMID: 9043075
- PMCID: PMC2041934
- DOI: 10.1242/dev.124.3.603
A single morphogenetic field gives rise to two retina primordia under the influence of the prechordal plate
Abstract
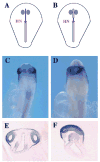
Two bilaterally symmetric eyes arise from the anterior neural plate in vertebrate embryos. An interesting question is whether both eyes share a common developmental origin or they originate separately. We report here that the expression pattern of a new gene ET reveals that there is a single retina field which resolves into two separate primordia, a suggestion supported by the expression pattern of the Xenopus Pax-6 gene. Lineage tracing experiments demonstrate that retina field resolution is not due to migration of cells in the median region to the lateral parts of the field. Removal of the prechordal mesoderm led to formation of a single retina both in chick embryos and in Xenopus explants. Transplantation experiments in chick embryos indicate that the prechordal plate is able to suppress Pax-6 expression. Our results provide direct evidence for the existence of a single retina field, indicate that the retina field is resolved by suppression of retina formation in the median region of the field, and demonstrate that the prechordal plate plays a primary signaling role in retina field resolution.
Figures









References
-
- Adelmann HB. Experimental studies on the development of the eye. I The effect of the removal of median and lateral areas of the anterior end of the urodelan neural plate on the development of the eyes (Triton teniatus and Amblystoma punctatum) J Exp Zool. 1929a;54:249–290.
-
- Adelmann HB. Experimental studies on the development of the eye. II The eye-forming potencies of the median portions of the urodelan neural plate (Triton teniatus and Amblystoma punctatum) J Exp Zool. 1929b;54:291–317.
-
- Adelmann HB. Experimental studies on the development of the eye. III The effect of the substrate (‘unterlagerung’) on the heterotopic development of median and lateral strips of the anterior end of the neural plate of Amblystoma. J Exp Zool. 1930;57:223–281.
-
- Adelmann HB. A study of cyclopia in Amblystoma punctatum, with special reference to the mesoderm. J Exp Zool. 1934;67:217–281.
-
- Adelmann HB. The problem of cyclopia. Pt I. Quar Rev Biol. 1936a;11:161–182.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

