alpha-MSH modulates local and circulating tumor necrosis factor-alpha in experimental brain inflammation
- PMID: 9045742
- PMCID: PMC6793749
- DOI: 10.1523/JNEUROSCI.17-06-02181.1997
alpha-MSH modulates local and circulating tumor necrosis factor-alpha in experimental brain inflammation
Abstract
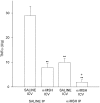
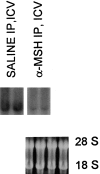
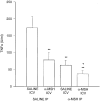
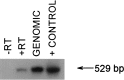
Tumor necrosis factor (TNF-alpha) underlies pathological processes and functional disturbances in acute and chronic neurological disease and injury. The neuroimmunomodulatory peptide alpha-MSH modulates actions and production of proinflammatory cytokines including TNF-alpha, but there is no prior evidence that it alters TNF-alpha induced within the brain. To test for this potential influence of the peptide, TNF-alpha was induced centrally by local injection of bacterial lipopolysaccharide (LPS). alpha-MSH given once i.c.v. with LPS challenge, twice daily intraperitoneally (i.p.) for 5 d between central LPS injections, or both i.p. and centrally, inhibited production of TNF-alpha within brain tissue. Inhibition of TNF-alpha protein formation by alpha-MSH was confirmed by inhibition of TNF-alpha mRNA. Plasma TNF-alpha concentration was elevated markedly after central LPS, indicative of an augmented peripheral host response induced by the CNS signal. The increase was inhibited by alpha-MSH treatments, in relation to inhibition of central TNF-alpha. Presence within normal mouse brain of mRNA for the alpha-MSH receptor MC-1 suggests that the inhibitory effects of alpha-MSH on brain and plasma TNF-alpha might be mediated by this receptor subtype. The inhibitory effect of alpha-MSH on brain TNF-alpha did not depend on circulating factors because the effect also occurred in brain tissue in vitro. This indicates that alpha-MSH can act directly on brain cells to inhibit their production of TNF-alpha. If central TNF-alpha contributes to pathology in CNS disease and injury, and promotes inflammation in the periphery, agents that act on brain alpha-MSH receptors should decrease the pathological TNF-alpha reaction and promote tissue survival.
Figures
References
-
- Catania A, Rajora N, Capsoni F, Minonzio F, Star RA, Lipton JM. The neuropeptide α-MSH has specific receptors on neutrophils and reduces chemotaxis in vitro. Peptides. 1996;17:675–679. - PubMed
-
- Ceriani G, Macaluso A, Catania A, Lipton JM. Central neurogenic antiinflammatory action of α-MSH: modulation of peripheral inflammation induced by cytokines and other mediators of inflammation. Neuroendocrinology. 1994;59:138–143. - PubMed
-
- Dulaney R, Macaluso A, Woerner J, Hiltz ME, Catania A, Lipton JM. Changes in periperal inflammation induced by the central actions of an α-MSH analog and of endogenous pyrogen. Prog Neuroendocrin Immunol. 1992;5:179–186.
-
- Dulderian SJ, Kilpatrick L, Costarino AT, Jr, McCawley L, Fein J, Corcoran L, Zirin S, Harris MC. Cytokine elevations in infants with bacterial and aseptic meningitis. J Pediatr. 1995;125:872–876. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
