Acetylcholine, outer hair cell electromotility, and the cochlear amplifier
- PMID: 9045745
- PMCID: PMC6793750
- DOI: 10.1523/JNEUROSCI.17-06-02212.1997
Acetylcholine, outer hair cell electromotility, and the cochlear amplifier
Abstract
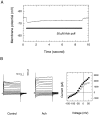
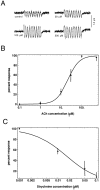
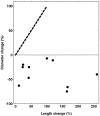
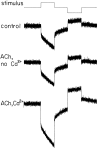
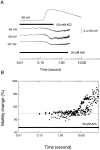
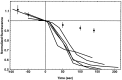
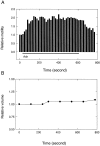
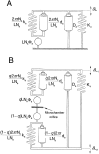
The dominant efferent innervation of the cochlea terminates on outer hair cells (OHCs), with acetylcholine (ACh) being its principal neurotransmitter. OHCs respond with a somatic shape change to alterations in their membrane potential, and this electromotile response is believed to provide mechanical feedback to the basilar membrane. We examine the effects of ACh on electromotile responses in isolated OHCs and attempt to deduce the mechanism of ACh action. Axial electromotile amplitude and cell compliance increase in the presence of the ligand. This response occurs with a significantly greater latency than membrane current and potential changes attributable to ACh and is contemporaneous with Ca2+ release from intracellular stores. It is likely that increased axial compliance largely accounts for the increase in motility. The mechanical responses are probably related to a recently demonstrated slow efferent effect. The implications of the present findings related to commonly assumed efferent behavior in vivo are considered.
Figures



References
-
- Allen JB. Modeling the noise damaged cochlea. In: Dallos P, Geisler CD, Matthews JW, Ruggero MA, Steele CR, editors. The mechanics and biophysics of hearing. Springer; Berlin: 1990. pp. 324–332.
-
- Ashmore JF. Transducer motor coupling in cochlear outer hair cells. In: Wilson JP, Kemp DT, editors. Mechanics of hearing. Plenum; New York: 1989. pp. 107–113.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
