Mitochondrial participation in the intracellular Ca2+ network
- PMID: 9049249
- PMCID: PMC2132502
- DOI: 10.1083/jcb.136.4.833
Mitochondrial participation in the intracellular Ca2+ network
Abstract
Calcium can activate mitochondrial metabolism, and the possibility that mitochondrial Ca2+ uptake and extrusion modulate free cytosolic [Ca2+] (Cac) now has renewed interest. We use whole-cell and perforated patch clamp methods together with rapid local perfusion to introduce probes and inhibitors to rat chromaffin cells, to evoke Ca2+ entry, and to monitor Ca2+-activated currents that report near-surface [Ca2+]. We show that rapid recovery from elevations of Cac requires both the mitochondrial Ca2+ uniporter and the mitochondrial energization that drives Ca2+ uptake through it. Applying imaging and single-cell photometric methods, we find that the probe rhod-2 selectively localizes to mitochondria and uses its responses to quantify mitochondrial free [Ca2+] (Cam). The indicated resting Cam of 100-200 nM is similar to the resting Cac reported by the probes indo-1 and Calcium Green, or its dextran conjugate in the cytoplasm. Simultaneous monitoring of Cam and Cac at high temporal resolution shows that, although Cam increases less than Cac, mitochondrial sequestration of Ca2+ is fast and has high capacity. We find that mitochondrial Ca2+ uptake limits the rise and underlies the rapid decay of Cac excursions produced by Ca2+ entry or by mobilization of reticular stores. We also find that subsequent export of Ca2+ from mitochondria, seen as declining Cam, prolongs complete Cac recovery and that suppressing export of Ca2+, by inhibition of the mitochondrial Na+/ Ca2+ exchanger, reversibly hastens final recovery of Cac. We conclude that mitochondria are active participants in cellular Ca2+ signaling, whose unique role is determined by their ability to rapidly accumulate and then release large quantities of Ca2+.
Figures
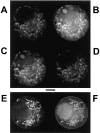
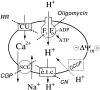

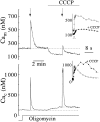





References
-
- Agard DA, Hiraoka Y, Shaw P, Sedat JW. Fluorescence microscopy in three dimensions. Methods Cell Biol. 1989;30:353–377. - PubMed
-
- Budd SL, Nichols DG. A reevaluation of the role of mitochondria in neuronal Ca2+homeostasis. J Neurochem. 1996;66:403–411. - PubMed
-
- Burnier M, Centeno G, Burki E, Brunner HR. Confocal microscopy to analyze cytosolic and nuclear calcium in cultured vascular muscle cells. Am J Physiol. 1994;266:C1118–C1127. - PubMed
-
- Carafoli E. The calcium cycle of mitochondria. FEBS Lett. 1979;104:1–5. - PubMed
-
- Chen, H., J.R. Swedlow, M. Gote, J.W. Sedat, and D.A. Agard. 1995. The collection, processing, and display of digital three-dimensional images of biological specimens. In Handbook of Biological Confocal Microscopy. J.B.Pawley, editor. Plenum Press, NY. 197–210.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

