Induction of neurite outgrowth through contactin and Nr-CAM by extracellular regions of glial receptor tyrosine phosphatase beta
- PMID: 9049255
- PMCID: PMC2132488
- DOI: 10.1083/jcb.136.4.907
Induction of neurite outgrowth through contactin and Nr-CAM by extracellular regions of glial receptor tyrosine phosphatase beta
Abstract
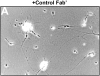
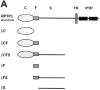
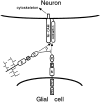
Receptor protein tyrosine phosphatase beta (RPTPbeta) is expressed as soluble and receptor forms with common extracellular regions consisting of a carbonic anhydrase domain (C), a fibronectin type III repeat (F), and a unique region called S. We showed previously that a recombinant Fc fusion protein with the C domain (beta C) binds to contactin and supports neuronal adhesion and neurite growth. As a substrate, betaCFS was less effective in supporting cell adhesion, but it was a more effective promoter of neurite outgrowth than betaCF. betaS had no effect by itself, but it potentiated neurite growth when mixed with betaCF. Neurite outgrowth induced by betaCFS was inhibited by antibodies against Nr-CAM and contactin, and these cell adhesion molecules formed a complex that bound betaCFS. NIH-3T3 cells transfected to express betaCFS on their surfaces induced neuronal differentiation in culture. These results suggest that binding of glial RPTPbeta to the contactin/Nr-CAM complex is important for neurite growth and neuronal differentiation.
Figures














References
-
- Alves F, Vogel W, Mossie K, Millauer B, Hofler H, Ullrich A. Distinct structural characteristics of discoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene. 1995;10:609–618. - PubMed
-
- Barnea G, Silvennoinen O, Shannan B, Honegger AM, Canoll PD, D'Estachio P, Levy JL, Laforgia S, Huebner K, Musacchio JM, et al. Identification of a carbonic anhydrase-like domain in the extracellular region of RPTP-γ defines a new subfamily of receptor tyrosine phosphatases. Mol Cell Biol. 1993;13:1497–1506. - PMC - PubMed
-
- Barnea G, Grumet M, Milev P, Silvennoinen O, Levy JB, Sap J, Schlessinger J. Receptor tyrosine phosphatase β is expressed in the form of a proteoglycan and binds to the extracellular matrix protein tenascin. J Biol Chem. 1994a;269:14349–14352. - PubMed
-
- Barnea G, Grumet M, Sap J, Margolis RU, Schlessinger J. Close similarity between a receptor-linked tyrosine phosphatase and a rat brain proteoglycan. Cell. 1994b;76:205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

