Cytohesin-1, a cytosolic guanine nucleotide-exchange protein for ADP-ribosylation factor
- PMID: 9050849
- PMCID: PMC19987
- DOI: 10.1073/pnas.94.5.1745
Cytohesin-1, a cytosolic guanine nucleotide-exchange protein for ADP-ribosylation factor
Abstract
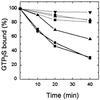
Cytohesin-1, a protein abundant in cells of the immune system, has been proposed to be a human homolog of the Saccharomyces cerevisiae Sec7 gene product, which is crucial in protein transport. More recently, the same protein has been reported to be a regulatory factor for the alphaLbeta2 integrin in lymphocytes. Overexpression of human or yeast ADP-ribosylation factor (ARF) genes rescues yeast with Sec7 defects, restoring secretory pathway function. ARFs, 20-kDa guanine nucleotide-binding proteins initially identified by their ability to stimulate cholera toxin ADP-ribosyltransferase activity and now recognized as critical components in intracellular vesicular transport, exist in an inactive cytosolic form with GDP bound (ARF-GDP). Interaction with a guanine nucleotide-exchange protein (GEP) accelerates exchange of GDP for GTP, producing the active ARF-GTP. Both soluble and particulate GEPs have been described. To define better the interaction between ARF and Sec7-related proteins, effects of cytohesin-1, synthesized in Escherichia coli, on ARF activity were evaluated. Cytohesin-1 enhanced binding of 35S-labeled guanosine 5'-[gamma-thio]triphosphate [35S]GTP[gammaS] or [3H]GDP to ARF purified from bovine brain (i.e., it appeared to function as an ARF-GEP). Addition of cytohesin-1 to ARF3 with [35S]GTP[gammaS] bound, accelerated [35S]GTP[gammaS] release to a similar degree in the presence of unlabeled GDP or GTP[gammaS] and to a lesser degree with GDP[betaS]; release was negligible without added nucleotide. Cytohesin-1 also increased ARF1 binding to a Golgi fraction, but its effect was not inhibited by brefeldin A (BFA), a drug that reversibly inhibits Golgi function. In this regard, it differs from a recently reported BFA-sensitive ARF-GEP that contains a Sec7 domain.
Figures




References
-
- Liu L, Pohajdak B. Biochim Biophys Acta. 1992;1132:75–78. - PubMed
-
- Kolanus W, Nagel W, Schiller B, Zeitlmann L, Godar S, Stockinger H, Seed B. Cell. 1996;86:233–242. - PubMed
-
- Shevell D E, Leu W M, Gillmor C S, Xia G, Feldmann K A, Chua N H. Cell. 1994;77:1051–1062. - PubMed
-
- Musacchio A, Gibson T, Rice P, Thompson J, Saraste M. Trends Biochem Sci. 1993;18:343–348. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

