Transcriptional arrest: Escherichia coli RNA polymerase translocates backward, leaving the 3' end of the RNA intact and extruded
- PMID: 9050851
- PMCID: PMC19989
- DOI: 10.1073/pnas.94.5.1755
Transcriptional arrest: Escherichia coli RNA polymerase translocates backward, leaving the 3' end of the RNA intact and extruded
Abstract
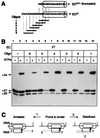
RNA polymerase (RNAP) may become arrested during transcript elongation when ternary complexes remain intact but further RNA synthesis is blocked. Using a combination of DNA and RNA footprinting techniques, we demonstrate that the loss of catalytic activity upon arrest of Escherichia coli RNAP is accompanied by an isomerization of the ternary complex in which the enzyme disengages from the 3' end of the transcript and moves backward along the DNA with concomitant reverse threading of the intact RNA through the enzyme. The reversal of RNAP brings the active center to the internal RNA position and thereby it represents a step in factor-facilitated transcript cleavage. Secondary structure elements or the 5' end of the transcript can prevent the isomerization by blocking the RNA threading. The described novel property of RNAP has far-reaching implications for the understanding of the elongation mechanism and gene regulation.
Figures




References
-
- Arndt K M, Chamberlin M J. J Mol Biol. 1990;213:79–108. - PubMed
-
- Krummel B, Chamberlin M J. J Mol Biol. 1992;225:221–234. - PubMed
-
- Borukhov S, Sagitov V, Goldfarb A. Cell. 1993;72:459–466. - PubMed
-
- Reines D, Chamberlin M J, Kane C M. J Biol Chem. 1989;264:10799–10809. - PubMed
-
- Nudler E, Kashlev M, Nikiforov V, Goldfarb A. Cell. 1995;81:351–357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

