Hepatitis B virus (HBV) envelope glycoproteins vary drastically in their sensitivity to glycan processing: evidence that alteration of a single N-linked glycosylation site can regulate HBV secretion
- PMID: 9050863
- PMCID: PMC20001
- DOI: 10.1073/pnas.94.5.1822
Hepatitis B virus (HBV) envelope glycoproteins vary drastically in their sensitivity to glycan processing: evidence that alteration of a single N-linked glycosylation site can regulate HBV secretion
Abstract
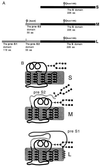
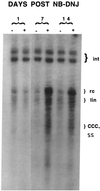
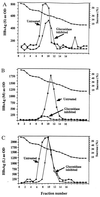
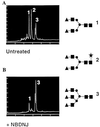
The role of N-linked glycosylation and glycan trimming in the function of glycoproteins remains a central question in biology. Hepatitis B virus specifies three glycoproteins (L, M, and S) that are derived from alternate translation of the same ORF. All three glycoproteins contain a common N-glycosylation site in the S domain while M possesses an additional N-glycosylation site at its amino terminus. In the presence of N-butyl-deoxnojirimycin (an inhibitor of alpha-glucosidase) virions and the M protein are surprisingly retained. Preliminary evidence suggests that the retained M protein is hyperglucosylated and localized to lysosomal vesicles. In contrast, the S and L proteins are secreted, and their glycosylation state is unaffected by the presence of the inhibitor. Site-directed mutagenesis provides evidence that virion secretion requires the glycosylation sequon in the pre-S2 domain of M. This highlights the potential role of the M protein oligosaccharide as a therapeutic target.
Figures
References
-
- Heermann K H, Gerlich W H. In: Molecular Biology of HBV. Maclachlan A, editor. Boca Raton, FL: CRC; 1992. pp. 109–143.
-
- Ganem D. Curr Top Microbiol Immunol. 1991;168:61–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

