Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development
- PMID: 9050868
- PMCID: PMC20006
- DOI: 10.1073/pnas.94.5.1852
Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development
Abstract
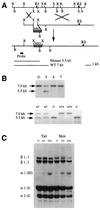
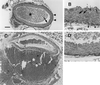
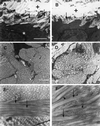
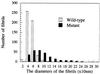
Type III collagen is a fibrillar forming collagen comprising three alpha1(III) chains and is expressed in early embryos and throughout embryogenesis. In the adult, type III collagen is a major component of the extracellular matrix in a variety of internal organs and skin. Mutations in the COL3A1 gene have been implicated as a cause of type IV Ehlers-Danlos syndrome, a disease leading to aortic rupture in early adult life. To directly study the role of Col3a1 in development and disease, we have inactivated the Col3a1 gene in embryonic stem cells by homologous recombination. The mutated allele was transmitted through the mouse germ line and homozygous mutant animals were derived from heterozygous intercrosses. About 10% of the homozygous mutant animals survived to adulthood but have a much shorter life span compared with wild-type mice. The major cause of death of mutant mice was rupture of the major blood vessels, similar to patients with type IV Ehlers-Danlos syndrome. Ultrastructural analysis of tissues from mutant mice revealed that type III collagen is essential for normal collagen I fibrillogenesis in the cardiovascular system and other organs.
Figures

References
-
- Prockop D J, Kivirikko K I. N Engl J Med. 1984;311:376–386. - PubMed
-
- Kuivaniemi H, Tromp G, Bergfeld W F, Kay M, Helm T N. J Invest Dermatol. 1995;105:352–356. - PubMed
-
- Olsen B R. Experientia. 1995;51:194–195. - PubMed
-
- Wong R S, Follis F M, Shively B K, Wernly J A. Ann Thorac Surg. 1995;60:1439–1443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

