syk kinase activation by a src kinase-initiated activation loop phosphorylation chain reaction
- PMID: 9050880
- PMCID: PMC20018
- DOI: 10.1073/pnas.94.5.1919
syk kinase activation by a src kinase-initiated activation loop phosphorylation chain reaction
Abstract
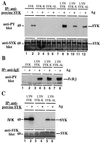
Activation of the syk tyrosine kinase occurs almost immediately following engagement of many types of antigen receptors, including Fc receptors, but the mechanism through which syk is activated is currently unclear. Here we demonstrate that Fc receptor-induced syk activation occurs as the result of phosphorylation of the syk activation loop by both src family kinases and other molecules of activated syk, suggesting that syk activation occurs as the result of a src kinase-initiated activation loop phosphorylation chain reaction. This type of activation mechanism predicts that syk activation would exhibit exponential kinetics, providing a potential explanation for its rapid and robust activation by even weak antigen receptor stimuli. We propose that a similar mechanism may be responsible for generating rapid activation of other cytoplasmic tyrosine kinases, such as those of the Bruton tyrosine kinase/tec family, as well.
Figures


References
-
- Ravetch J V, Kinet J P. Annu Rev Immunol. 1991;9:457–492. - PubMed
-
- Metzger H. Immunol Rev. 1992;125:37–48. - PubMed
-
- Weiss A, Littman D R. Cell. 1994;76:263–274. - PubMed
-
- Cambier J C. J Immunol. 1995;155:3281–3285. - PubMed
-
- Benhamou M, Ryba N J, Kihara H, Nishikata H, Siraganian R P. J Biol Chem. 1993;268:23318–23324. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

