Glutathione deficiency is associated with impaired survival in HIV disease
- PMID: 9050888
- PMCID: PMC20026
- DOI: 10.1073/pnas.94.5.1967
Glutathione deficiency is associated with impaired survival in HIV disease
Abstract
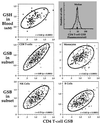
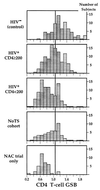
Glutathione (GSH), a cysteine-containing tripeptide, is essential for the viability and function of virtually all cells. In vitro studies showing that low GSH levels both promote HIV expression and impair T cell function suggested a link between GSH depletion and HIV disease progression. Clinical studies presented here directly demonstrate that low GSH levels predict poor survival in otherwise indistinguishable HIV-infected subjects. Specifically, we show that GSH deficiency in CD4 T cells from such subjects is associated with markedly decreased survival 2-3 years after baseline data collection (Kaplan-Meier and logistic regression analyses, P < 0.0001 for both analyses). This finding, supported by evidence demonstrating that oral administration of the GSH prodrug N-acetylcysteine replenishes GSH in these subjects and suggesting that N-acetylcysteine administration can improve their survival, establishes GSH deficiency as a key determinant of survival in HIV disease. Further, it argues strongly that the unnecessary or excessive use of acetaminophen, alcohol, or other drugs known to deplete GSH should be avoided by HIV-infected individuals.
Figures

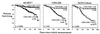


References
-
- Vina J, Vinar J R, Saez G T. Life Chem Rep. 1986;4:1–35.
-
- Dolphin D, Avramovic O, Poulson R. Glutathione: Coenzymes and Cofactors. New York: Wiley; 1989. Vols. 3A and 3B.
-
- Taniguchi N, Higashi T, Sakamoto Y, Meister A. Glutathione Centennial. New York: Academic; 1989.
-
- Poot M, Teubert H, Rabinovitch P S, Kavanagh T J. J Cell Physiol. 1995;163:555–560. - PubMed
-
- Adamson D C, Wildemann B, Sasaki M, Glass J D, McArthur J c, Christov V I, Dawson T M, Dawson V L. Science. 1996;274:1917–1919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

