Inhibition of interleukin 1beta converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage
- PMID: 9050895
- PMCID: PMC20033
- DOI: 10.1073/pnas.94.5.2007
Inhibition of interleukin 1beta converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage
Abstract
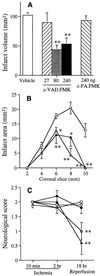
The interleukin 1beta converting enzyme (ICE) family plays a pivotal role in programmed cell death and has been implicated in stroke and neurodegenerative diseases. During reperfusion after filamentous middle cerebral artery occlusion, ICE-like cleavage products and tissue immunoreactive interleukin 1beta (IL-1beta) levels increased in ischemic mouse brain. Ischemic injury decreased after intracerebroventricular injections of ICE-like protease inhibitors, N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (z-VAD.FMK), acetyl-Tyr-Val-Ala-Asp-chloromethylketone, or a relatively selective inhibitor of CPP32-like caspases, N-benzyloxycarbonyl-Asp-Glu-Val-Asp-fluoromethylketone, but not a cathepsin B inhibitor, N-benzyloxycarbonyl-Phe-Ala-fluoromethylketone. z-VAD.FMK decreased ICE-like cleavage products and tissue immunoreactive IL-1beta levels in ischemic mouse brain and reduced tissue damage when administered to rats as well. Only z-VAD.FMK and acetyl-Tyr-Val-Ala-Asp-chloromethylketone reduced brain swelling, and N-benzyloxycarbonyl-Asp-Glu-Val-Asp-fluoromethylketone did not attenuate the ischemia-induced increase in tissue IL-1beta levels. The three cysteine protease inhibitors significantly improved behavioral deficits, thereby showing that functional recovery of ischemic neuronal tissue can follow blockade of enzymes associated with apoptotic cell death. Finally, we examined the effect of z-VAD.FMK on excitotoxicity and found that it protected against alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate-induced or to a lesser extent N-methyl-D-aspartate-induced excitotoxic brain damage. Thus, ICE-like and CPP32-like caspases contribute to mechanisms of cell death in ischemic and excitotoxic brain injury and provide therapeutic targets for stroke and neurodegenerative brain damage.
Figures



References
-
- Ellis R E, Yuan J, Horvitz H R. Annu Rev Cell Biol. 1991;7:663–698. - PubMed
-
- Yuan J, Horvitz H R. Dev Biol. 1990;138:33–41. - PubMed
-
- Yuan J, Shaham S, Ledoux S, Ellis H M, Horvitz H R. Cell. 1993;75:641–652. - PubMed
-
- Li Y, Chopp M, Jiang N, Yao F, Zaloga C. J Cereb Blood Flow Metab. 1995;15:389–397. - PubMed
-
- Charriaut-Marlangue C, Margaill I, Represa A, Popovici T, Plotkine M, Ben-Ari Y. J Cereb Blood Flow Metab. 1996;16:186–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

