The presenilin 2 mutation (N141I) linked to familial Alzheimer disease (Volga German families) increases the secretion of amyloid beta protein ending at the 42nd (or 43rd) residue
- PMID: 9050898
- PMCID: PMC20036
- DOI: 10.1073/pnas.94.5.2025
The presenilin 2 mutation (N141I) linked to familial Alzheimer disease (Volga German families) increases the secretion of amyloid beta protein ending at the 42nd (or 43rd) residue
Abstract
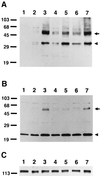
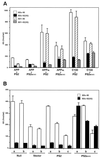
To gain insights into the significance of presenilins (PS) in the pathogenetic mechanisms of early-onset familial Alzheimer disease (FAD), we expressed cDNAs for wild-type PS2 and PS2 with the Volga German (N141I) mutation in cultured cells and then examined the metabolism of the transfected proteins and their effect on the C-terminal properties of secreted amyloid beta protein (A beta). PS2 was identified as a 50- to 55-kDa protein, which was cleaved to produce N-terminal fragments of 35-40 kDa and C-terminal fragments of 19-23 kDa. The Volga German (N141I) mutation did not cause any significant change in the metabolism of PS2. COS-1 cells doubly transfected with cDNAs for N141I mutant PS2 and human beta-amyloid precursor protein (betaAPP) or a C-terminal fragment thereof, as well as mouse Neuro2a neuroblastoma cells stably transfected with N141I mutant PS2 alone, secreted 1.5- to 10-fold more A beta ending at residues 42 (or 43) [A beta42(43)] compared with those expressing the wild-type PS2. These results strongly suggest that the PS2 mutation (N141I) linked to FAD alters the metabolism of A beta/betaAPP to foster the production of the form of A beta that most readily deposits in amyloid plaques. Thus, mutant PS2 may lead to AD by altering the metabolism of A beta/betaAPP.
Figures

Comment in
-
The Alzheimer family of diseases: many etiologies, one pathogenesis?Proc Natl Acad Sci U S A. 1997 Mar 18;94(6):2095-7. doi: 10.1073/pnas.94.6.2095. Proc Natl Acad Sci U S A. 1997. PMID: 9122152 Free PMC article. Review. No abstract available.
References
-
- Selkoe D J. J Biol Chem. 1996;271:18295–18298. - PubMed
-
- Goate A, Chartier-Harlin M-C, Mullan M, Brown J, Crawford F, et al. Nature (London) 1991;349:704–706. - PubMed
-
- Mullan M, Crawford F, Axelman K, Houldin H, Lilius L, Winblad B, Lannfelt L. Nat Genet. 1992;1:345–347. - PubMed
-
- Citron M, Oltersdolf T, Haass C, McConlogue L, Hung A Y, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe D J. Nature (London) 1992;360:672–674. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

