Pore size of the malaria parasite's nutrient channel
- PMID: 9050902
- PMCID: PMC20040
- DOI: 10.1073/pnas.94.5.2045
Pore size of the malaria parasite's nutrient channel
Abstract
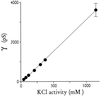
The malaria parasite, Plasmodium falciparum, requires large amounts of nutrients to sustain its rapid growth within the human red blood cell. A recently identified ion channel on the surface of the intraerythrocytic parasite may provide direct access to these nutrients in the red blood cell cytosol. Evidence supporting this role was obtained by incorporating this channel into planar lipid bilayers. In bilayers, this channel has conductance and gating properties identical to the in situ channel, passes soluble macromolecules of up to 1400 Da, and functions as a high capacity, low affinity molecular sieve. These properties, remarkably similar to those of a pore on Toxoplasma gondii (another protozoan parasite causing human disease), suggest a novel class of channels used by these intracellular parasites to acquire nutrients from host cytosol.
Figures



References
-
- Pouvelle B, Spiegel R, Hsiao L, Howard R J, Morris R L, Thomas A P, Taraschi T F. Nature (London) 1991;353:73–75. - PubMed
-
- Desai S A, Krogstad D J, McCleskey E W. Nature (London) 1993;362:643–646. - PubMed
-
- Ginsburg H. Biochem Pharmacol. 1994;48:1847–1856. - PubMed
-
- Desai S A. Parasitol Today. 1994;10:24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

