Nitric oxide synthase type II expression by different cell types in MHV-JHM encephalitis suggests distinct roles for nitric oxide in acute versus persistent virus infection
- PMID: 9058755
- PMCID: PMC7119606
- DOI: 10.1016/s0165-5728(96)00159-2
Nitric oxide synthase type II expression by different cell types in MHV-JHM encephalitis suggests distinct roles for nitric oxide in acute versus persistent virus infection
Abstract
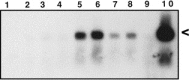
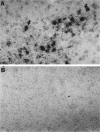
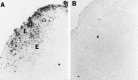
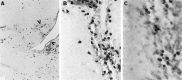
Intranasal inoculation with mouse hepatitis virus strain JHM (MHV-JHM) results in acute meningoencephalitis. We found NOS II mRNA expression in brains of acutely infected animals on days 5 through 7 after infection. In situ hybridization and immunohistochemistry demonstrated NOS II message and protein in infiltrating macrophages. Persistent infection with MHV-JHM results in chronic demyelinating encephalomyelitis. NOS II mRNA was detected in persistently infected spinal cords. In situ hybridization and immunohistochemistry showed expression of NOS II in astrocytes in and around demyelinated lesions. These results suggest the role of NO release in acute versus persistent infection with this virus, and its contribution to the resulting pathology, may be different.
Figures

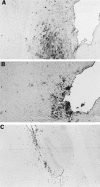
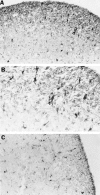
References
-
- Adams, L.B., Hibbs, J.B., Taintor, R.R. and Krahenbuhl, J.L. (1990) Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J. Immunol. 144, 2725–2729. - PubMed
-
- Benveniste, E.N. (1992) Inflammatory cytokines within the central nervous system:sources, function, and mechanism of action. Am. J. Physiol. 263, C1–C16. - PubMed
-
- Bauer, J., Berkenbosch, F., Van Dam, A. and Dijkstra, C.D. (1993) Demonstration of interleukin-1b in Lewis rat brain during experimental allergic encephalomyelitis by immunocytochemistry at the light and ultrastructural level. J. Neuroimmunol. 48, 13–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

