Identification of protein transport complexes in the chloroplastic envelope membranes via chemical cross-linking
- PMID: 9060464
- PMCID: PMC2132478
- DOI: 10.1083/jcb.136.5.983
Identification of protein transport complexes in the chloroplastic envelope membranes via chemical cross-linking
Abstract
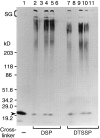
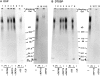
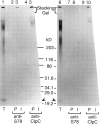
Transport of cytoplasmically synthesized proteins into chloroplasts uses an import machinery present in the envelope membranes. To identify the components of this machinery and to begin to examine how these components interact during transport, chemical cross-linking was performed on intact chloroplasts containing precursor proteins trapped at a particular stage of transport by ATP limitation. Large cross-linked complexes were observed using three different reversible homobifunctional cross-linkers. Three outer envelope membrane proteins (OEP86, OEP75, and OEP34) and one inner envelope membrane protein (IEP110), previously reported to be involved in protein import, were identified as components of these complexes. In addition to these membrane proteins, a stromal member of the hsp100 family, ClpC, was also present in the complexes. We propose that ClpC functions as a molecular chaperone, cooperating with other components to accomplish the transport of precursor proteins into chloroplasts. We also propose that each envelope membrane contains distinct translocation complexes and that a portion of these interact to form contact sites even in the absence of precursor proteins.
Figures
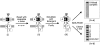

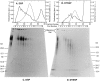
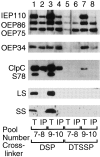
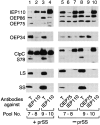
References
-
- Bruce, B.D., S. Perry, J. Froehlich, and K. Keegstra. 1994. In vitro import of proteins into chloroplasts. In Plant Molecular Biology Manual, J1. S.B. Gelvin and R.A. Schilperoort, editors. Kluwer Academic Publishers, Boston MA. 1–15.
-
- de Boer AD, Weisbeek PJ. Chloroplast protein topogenesis: import, sorting and assembly. Biochim Biophys Acta. 1991;1071:221–253. - PubMed

