LI-cadherin-mediated cell-cell adhesion does not require cytoplasmic interactions
- PMID: 9060475
- PMCID: PMC2132471
- DOI: 10.1083/jcb.136.5.1109
LI-cadherin-mediated cell-cell adhesion does not require cytoplasmic interactions
Abstract
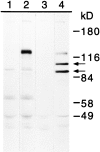
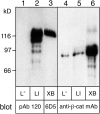
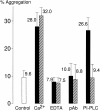
The adhesive function of classical cadherins depends on the association with cytoplasmic proteins, termed catenins, which serve as a link between cadherins and the actin cytoskeleton. LI-cadherin, a structurally different member of the cadherin family, mediates Ca2+-dependent cell-cell adhesion, although its markedly short cytoplasmic domain exhibits no homology to this highly conserved region of classical cadherins. We now examined whether the adhesive function of LI-cadherin depends on the interaction with catenins, the actin cytoskeleton or other cytoplasmic components. In contrast to classical cadherins, LI-cadherin, when expressed in mouse L cells, was neither associated with catenins nor did it induce an upregulation of beta-catenin. Consistent with these findings, LI-cadherin was not resistant to detergent extraction and did not induce a reorganization of the actin cytoskeleton. However, LI-cadherin was still able to mediate Ca2+-dependent cell-cell adhesion. To analyze whether this function requires any interaction with proteins other than catenins, a glycosyl phosphatidylinositol-anchored form of LI-cadherin (LI-cadherin(GPI)) was constructed and expressed in Drosophila S2 cells. The mutant protein was able to induce Ca2+-dependent, homophilic cell-cell adhesion, and its adhesive properties were indistinguishable from those of wild type LI-cadherin. These findings indicate that the adhesive function of LI-cadherin is independent of any interaction with cytoplasmic components, and consequently should not be sensitive to regulatory mechanisms affecting the binding of classical cadherins to catenins and to the cytoskeleton. Thus, we postulate that the adhesive function of LI-cadherin is complementary to that of coexpressed classical cadherins ensuring cell-cell contacts even under conditions that downregulate the function of classical cadherins.
Figures








References
-
- Behrens J, Vakaet L, Friis R, Winterhager E, Van RF, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. - PMC - PubMed
-
- Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochem Biophys Acta. 1994;1198:11–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

