Neuroprotective action of cycloheximide involves induction of bcl-2 and antioxidant pathways
- PMID: 9060477
- PMCID: PMC2132476
- DOI: 10.1083/jcb.136.5.1137
Neuroprotective action of cycloheximide involves induction of bcl-2 and antioxidant pathways
Abstract
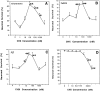
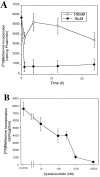
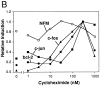
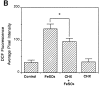
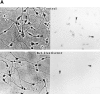
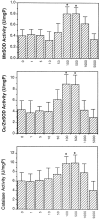
The ability of the protein synthesis inhibitor cycloheximide (CHX) to prevent neuronal death in different paradigms has been interpreted to indicate that the cell death process requires synthesis of "killer" proteins. On the other hand, data indicate that neurotrophic factors protect neurons in the same death paradigms by inducing expression of neuroprotective gene products. We now provide evidence that in embryonic rat hippocampal cell cultures, CHX protects neurons against oxidative insults by a mechanism involving induction of neuroprotective gene products including the antiapoptotic gene bcl-2 and antioxidant enzymes. Neuronal survival after exposure to glutamate, FeSO4, and amyloid beta-peptide was increased in cultures pretreated with CHX at concentrations of 50-500 nM; higher and lower concentrations were ineffective. Neuroprotective concentrations of CHX caused only a moderate (20-40%) reduction in overall protein synthesis, and induced an increase in c-fos, c-jun, and bcl-2 mRNAs and protein levels as determined by reverse transcription-PCR analysis and immunocytochemistry, respectively. At neuroprotective CHX concentrations, levels of c-fos heteronuclear RNA increased in parallel with c-fos mRNA, indicating that CHX acts by inducing transcription. Neuroprotective concentrations of CHX suppressed accumulation of H2O2 induced by FeSO4, suggesting activation of antioxidant pathways. Treatment of cultures with an antisense oligodeoxynucleotide directed against bcl-2 mRNA decreased Bcl-2 protein levels and significantly reduced the neuroprotective action of CHX, suggesting that induction of Bcl-2 expression was mechanistically involved in the neuroprotective actions of CHX. In addition, activity levels of the antioxidant enzymes Cu/Zn-superoxide dismutase, Mn-superoxide dismutase, and catalase were significantly increased in cultures exposed to neuroprotective levels of CHX. Our data suggest that low concentrations of CHX can promote neuron survival by inducing increased levels of gene products that function in antioxidant pathways, a neuroprotective mechanism similar to that used by neurotrophic factors.
Figures










References
-
- Aden U, Bona E, Hagberg H, Fredholm BB. Changes in c-fos mRNA in the neonatal rat brain following hypoxic ischemia. Neurosci Lett. 1994;180:91–95. - PubMed
-
- Allsopp TE, Kiselev S, Wyatt S, Davies AM. Role of Bcl-2 in the brain-derived neurotrophic factor survival response. Eur J Neurosci. 1995;7:1266–1272. - PubMed
-
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. - PubMed
-
- Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol. 1995;38:357–366. - PubMed
-
- Behl C, Davis J, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid β protein toxicity. Cell. 1994;77:817–827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

