Intercircuit control of motor pattern modulation by presynaptic inhibition
- PMID: 9065486
- PMCID: PMC6573485
- DOI: 10.1523/JNEUROSCI.17-07-02247.1997
Intercircuit control of motor pattern modulation by presynaptic inhibition
Abstract
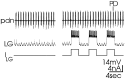
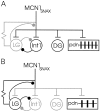
Rhythmically active neural networks can control the modulatory input that they receive via their synaptic effects onto modulatory neurons. This synaptic control of network modulation can occur presynaptically, at the axon terminals of the modulatory neuron. For example, in the crab stomatogastric ganglion (STG), a gastric mill network neuron presynaptically inhibits transmitter release from a modulatory projection neuron called modulatory commissural neuron 1. We showed previously that the gastric mill rhythm-timed presynaptic inhibition of the STG terminals of MCN1 is pivotal for enabling MCN1 to activate this rhythm. We also showed that MCN1 excites the pyloric rhythm within the STG. Here we show that, because MCN1 stimulation conjointly excites the gastric mill and pyloric rhythms, the gastric mill rhythm-timed presynaptic inhibition of MCN1 causes a rhythmic interruption in the MCN1-mediated excitation of the pyloric rhythm. Consequently, during each protraction phase of the gastric mill rhythm, presynaptic inhibition suppresses MCN1 excitation of the pyloric rhythm, thereby weakening the pyloric rhythm. During the retraction phase, presynaptic inhibition is absent and MCN1 elicits a faster, stronger, and modified pyloric rhythm. Thus, in addition to its role in enabling a neural circuit to regulate the modulatory transmission that it receives, presynaptic inhibition is also used effectively to rhythmically control the activity level of a distinct, but behaviorally related, neural circuit.
Figures







References
-
- Bal T, Nagy F, Moulins M. The pyloric central pattern generator in Crustacea: a set of conditional neuronal oscillators. J Comp Physiol [A] 1988;163:715–727.
-
- Bartos M, Nusbaum MP. Two proctolin/GABA neurons elicit distinct motor patterns from the same neural network. Soc Neurosci Abstr. 1996;22:1375.
-
- Blitz DM, Christie AE, Marder E, Nusbaum MP. Distribution and effects of tachykinin-like peptides in the stomatogastric nervous system of the crab, Cancer borealis. J Comp Neurol. 1995;354:282–294. - PubMed
-
- Chrachri A, Neil D. Interactions and synchronization between two abdominal motor systems in crayfish. J Neurophysiol. 1993;69:1371–1383. - PubMed
-
- Christie AE, Norris BJ, Coleman MJ, Marder E, Nusbaum MP. Neuropil arborizations and transmitter complement of a modulatory projection neuron. Soc Neurosci Abstr. 1993;19:931.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
