H+ permeation and pH regulation at a mammalian serotonin transporter
- PMID: 9065487
- PMCID: PMC6573487
- DOI: 10.1523/JNEUROSCI.17-07-02257.1997
H+ permeation and pH regulation at a mammalian serotonin transporter
Abstract
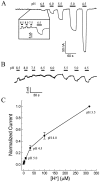
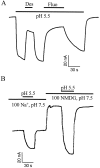
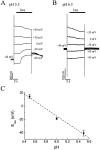
The rat serotonin transporter expressed in Xenopus oocytes displays an inward current in the absence of 5-HT when external pH is lowered to 6.5 or below. The new current differs from the leakage current described previously in two ways. (1) It is approximately 10-fold larger at pH 5 than the leakage current at pH 7.5 and reaches 1000 H+/sec per transporter at extremes of voltage and pH with no signs of saturation. (2) It is selective for H+ by reversal potential measurements. Similar H+-induced currents are also observed in several other ion-coupled transporters, including the GABA transporter, the dopamine transporter, and the Na+/glucose transporter. The high conductance and high selectivity of the H+-induced current suggest that protons may be conducted via a hydrogen-bonded chain (a "proton-wire mechanism") formed at least partially by side chains within the transporter. In addition, pH affects other conducting states of rat serotonin transporter. Acidic pH potentiates the 5-HT-induced, transport-associated current and inhibits the hyperpolarization-activated transient current. The dose-response relationships for these two effects suggest that two H+ binding sites, with pKa values close to 5.1 and close to 6.3, govern the potentiation of the 5-HT-induced current and the inhibition of the transient current, respectively. These results are important for developing structure-function models that explain permeation properties of neurotransmitter transporters.
Figures





References
-
- Billups B, Attwell D. Modulation of non-vesicular glutamate release by pH. Nature. 1996;379:171–174. - PubMed
-
- Cammack JN, Rakhilin SV, Schwartz EA. A GABA transporter operates asymmetrically and with variable stoichiometry. Neuron. 1994;13:949–960. - PubMed
-
- Csiba L, Paschen W, Hossmann K-A. A topographic quantitative method for measuring brain tissue pH under physiological and pathophysiological conditions. Brain Res. 1983;289:334–337. - PubMed
-
- Deamer DW, Nichols JW. Proton flux mechanisms in model and biological membranes. J Membr Biol. 1989;107:91–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
