Effects of charge state on fragmentation pathways, dynamics, and activation energies of ubiquitin ions measured by blackbody infrared radiative dissociation
- PMID: 9075403
- PMCID: PMC1434665
- DOI: 10.1021/ac960804q
Effects of charge state on fragmentation pathways, dynamics, and activation energies of ubiquitin ions measured by blackbody infrared radiative dissociation
Abstract
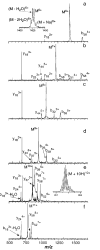
Blackbody infrared radiative dissociation spectra of the (M + 5H)5+ through (M + 11H)11+ ions of the protein ubiquitin (8.6 kDa) formed by electrospray ionization were measured in a Fourier-transform mass spectrometer. The 5+ ion dissociates exclusively by loss of water and/or ammonia, whereas the 11+ charge state dissociates only by formation of complementary y and b ions. These two processes are competitive for intermediate charge state ions, with the formation of y and b ions increasingly favored for the higher charge states. The y and b ions are formed by cleavage of the backbone amide bond on the C-terminal side of acidic residues exclusively, with cleavage adjacent to aspartic acid favored. Thermal unimolecular dissociation rate constants for the dissociation of each of these charge states were measured. From the temperature dependence of these rates, Arrhenius activation parameters in the rapid energy exchange limit are obtained. The activation energies (Ea) and preexponential factors (A) for the 5+, 8+, and 9+ ions are 1.2 eV and 10(12) s-1, respectively. These values for the 6+ and 7+ ions are 0.9-1.0 eV and 10(9) s-1, and those for the 10+ and 11+ ions are 1.6 eV and 10(16)-10(17) s-1. Thus, with the exception of the 5+ ion, the higher charge states of ubiquitin have larger dissociation activation energies than the lower charge states. The different A factors observed for production of y and b ions from different precursor charge states indicate that they are formed by different mechanisms, ranging from relatively complex rearrangements to direct bond cleavages. These results clearly demonstrate that the relative dissociation rates of large biomolecule ions by themselves are not necessarily a reliable indicator of their relative dissociation energies, even when similar fragment ions are formed.
Figures



Similar articles
-
Blackbody infrared radiative dissociation of oligonucleotide anions.J Am Soc Mass Spectrom. 1998 Nov;9(11):1117-24. doi: 10.1016/S1044-0305(98)00098-1. J Am Soc Mass Spectrom. 1998. PMID: 9794082 Free PMC article.
-
Tandem mass spectrometry of large biomolecule ions by blackbody infrared radiative dissociation.Anal Chem. 1996 Mar 1;68(5):859-66. doi: 10.1021/ac951038a. Anal Chem. 1996. PMID: 21619182
-
Dissociation energetics and mechanisms of leucine enkephalin (M + H)+ and (2M + X)+ ions (X = H, Li, Na, K, and Rb) measured by blackbody infrared radiative dissociation.J Am Soc Mass Spectrom. 1997 Aug;8(8):771-80. doi: 10.1016/S1044-0305(97)84129-3. J Am Soc Mass Spectrom. 1997. PMID: 16554908 Free PMC article.
-
Ion activation methods for tandem mass spectrometry.J Mass Spectrom. 2004 Oct;39(10):1091-112. doi: 10.1002/jms.703. J Mass Spectrom. 2004. PMID: 15481084 Review.
-
Activation of large ions in FT-ICR mass spectrometry.Mass Spectrom Rev. 2005 Mar-Apr;24(2):135-67. doi: 10.1002/mas.20012. Mass Spectrom Rev. 2005. PMID: 15389858 Review.
Cited by
-
Determination of the activation energy for unimolecular dissociation of a non-covalent gas-phase peptide: substrate complex by infrared multiphoton dissociation fourier transform ion cyclotron resonance mass spectrometry.J Am Soc Mass Spectrom. 2003 Nov;14(11):1282-9. doi: 10.1016/S1044-0305(03)00576-2. J Am Soc Mass Spectrom. 2003. PMID: 14597118
-
On the zwitterionic nature of gas-phase peptides and protein ions.PLoS Comput Biol. 2010 May 6;6(5):e1000775. doi: 10.1371/journal.pcbi.1000775. PLoS Comput Biol. 2010. PMID: 20463874 Free PMC article.
-
Protein conformation and supercharging with DMSO from aqueous solution.J Am Soc Mass Spectrom. 2011 Jul;22(7):1178-86. doi: 10.1007/s13361-011-0116-x. Epub 2011 Apr 19. J Am Soc Mass Spectrom. 2011. PMID: 21953100 Free PMC article.
-
Blackbody infrared radiative dissociation of oligonucleotide anions.J Am Soc Mass Spectrom. 1998 Nov;9(11):1117-24. doi: 10.1016/S1044-0305(98)00098-1. J Am Soc Mass Spectrom. 1998. PMID: 9794082 Free PMC article.
-
Electron capture by a hydrated gaseous peptide: effects of water on fragmentation and molecular survival.J Am Chem Soc. 2008 Sep 24;130(38):12680-9. doi: 10.1021/ja8022434. Epub 2008 Aug 30. J Am Chem Soc. 2008. PMID: 18761457 Free PMC article.
References
-
- Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Science. 1989;246:64–71. - PubMed
- Smith RD, Loo JA, Loo RRO, Busman M, Udseth HR. Mass Spectrom Rev. 1991;10:359–451.
-
- Hillenkamp F, Karas M, Beavis RC, Chait BT. Anal Chem. 1991. pp. 1193A–1203A. - PubMed
-
- Chen R, Cheng X, Mitchell DW, Hofstadler SA, Wu Q, Rockwood AL, Sherman MG, Smith RD. Anal Chem. 1995;67:1159–1163.
-
- O’Connor PB, Speir JP, Senko MW, Little DP, McLafferty FW. J Mass Spectrom. 1995;30:88–93.
- McLuckey SA, Habibi-Goudarzi S. J Am Chem Soc. 1993;115:12085–12095.
- Dongré AR, Somogyi A, Wysocki VH. J Mass Spectrom. 1996;31:339–350. - PubMed
- Hunt DF, Yates JR, III, Shabanowitz J, Winston S, Hauer CR. Proc Natl Acad Sci USA. 1986;83:6233–6237. - PMC - PubMed
-
- Huddleston MJ, Bean MF, Carr SA. Anal Chem. 1993;65:877–884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

